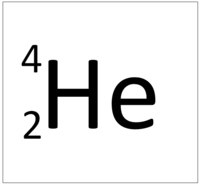Difference between revisions of "Relative Atomic Mass"
(→Examples) |
|||
| Line 31: | Line 31: | ||
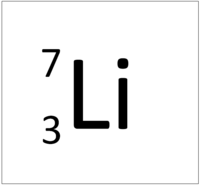
| style="height:20px; width:200px; text-align:center;" |[[Lithium]] has seven [[nucleon]]s so it has an '''atomic mass''' of 7. | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] has seven [[nucleon]]s so it has an '''atomic mass''' of 7. | ||
| style="height:20px; width:200px; text-align:center;" |[[Beryllium]] has nine [[nucleon]]s so it has an '''atomic mass''' of 9. | | style="height:20px; width:200px; text-align:center;" |[[Beryllium]] has nine [[nucleon]]s so it has an '''atomic mass''' of 9. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
| + | [[File:ElementTile.png|right|400px|thumb|An [[element]] tile showing the '''mass number'''.]] | ||
| + | The '''relative atomic mass''' or '''mass number''' of an [[element]] is the number of [[nucleon]]s ([[proton]]s + [[neutron]]s) in an [[atom]]. | ||
| + | The '''relative atomic mass''' in [[gram]]s is also the [[mass]] of one [[mole]] or [[Avagadro Constant|6.02x10<sup>23</sup>]] [[atom]]s of the [[element]]. | ||
| + | |||
| + | ===About Relative Atomic Mass=== | ||
| + | : The '''relative atomic mass''' in [[gram]]s on the [[Periodic Table]] tells the [[mass]] of a [[mole]] of the [[element]]. | ||
| + | : A [[mole]] of the [[element]] is [[Avagadro Constant|6.02x10<sup>23</sup>]] [[atom]]s of that [[element]]. | ||
| + | : Two [[atom]]s of the same [[element]] may have the same [[Atomic Number]] but a different [[Relative Atomic Mass]] depending on the number of [[neutron]]s in the [[nucleus]]. [[Element]]s with different '''mass numbers''' are called [[isotope]]s. | ||
| + | : The '''relative atomic mass''' is not affected by the number of [[electron]]s. | ||
| + | : Only the [[particle]]s in the [[nucleus]] affect the '''relative atomic mass'''. | ||
| + | |||
| + | ===Examples=== | ||
| + | |||
| + | {| class="wikitable" | ||
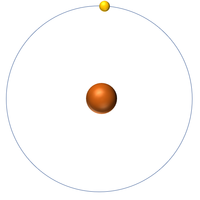
| + | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen''' | ||
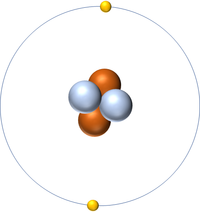
| + | | style="height:20px; width:200px; text-align:center;" |'''Helium''' | ||
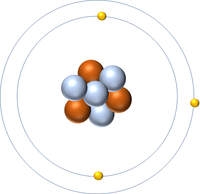
| + | | style="height:20px; width:200px; text-align:center;" |'''Lithium''' | ||
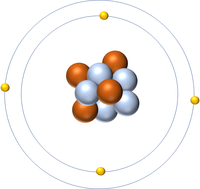
| + | | style="height:20px; width:200px; text-align:center;" |'''Beryllium''' | ||
| + | |- | ||
| + | |[[File:Hydrogen.png|center|200px]] | ||
| + | |[[File:Helium.png|center|200px]] | ||
| + | |[[File:Lithium.png|center|200px]] | ||
| + | |[[File:Beryllium.png|center|200px]] | ||
| + | |- | ||
| + | |[[File:HydrogenSymbol.png|center|200px]] | ||
| + | |[[File:HeliumSymbol.png|center|200px]] | ||
| + | |[[File:LithiumSymbol.png|center|200px]] | ||
| + | |[[File:BerylliumSymbol.png|center|200px]] | ||
| + | |- | ||
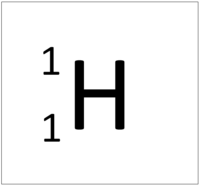
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] has one [[nucleon]] so it has an '''atomic mass''' of 1 and [[Avagadro Constant|6.02x10<sup>23</sup>]] (or 1 [[mole]] of) [[atom]]s of [[Hydrogen]] have a [[mass]] of 1g. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Helium]] has four [[nucleon]]s so it has an '''atomic mass''' of 4 and [[Avagadro Constant|6.02x10<sup>23</sup>]] (or 1 [[mole]] of) [[atom]]s of [[Helium]] have a [[mass]] of 4g. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] has seven [[nucleon]]s so it has an '''atomic mass''' of 7 and [[Avagadro Constant|6.02x10<sup>23</sup>]] (or 1 [[mole]] of) [[atom]]s of [[Lithium]] have a [[mass]] of 7g. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Beryllium]] has nine [[nucleon]]s so it has an '''atomic mass''' of 9 and [[Avagadro Constant|6.02x10<sup>23</sup>]] (or 1 [[mole]] of) [[atom]]s of [[Beryllium]] have a [[mass]] of 9g. | ||
|} | |} | ||
Revision as of 18:54, 2 January 2019
Contents
Key Stage 3
Meaning
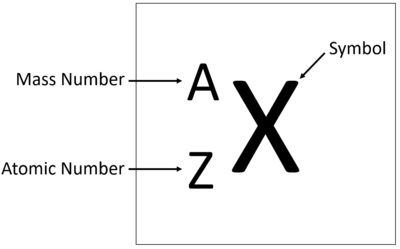
An element tile showing the mass number.
The Atomic Mass or mass number is the number of nucleons (protons + neutrons) in an atom.
About The Atomic Mass
- Two atoms of the same element may have the same Atomic Number but a different Atomic Mass depending on the number of neutrons in the nucleus. Elements with different mass numbers are called isotopes.
- The atomic mass is not affected by the number of electrons.
- Only the particles in the nucleus affect the atomic mass.
Examples
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has one nucleon so it has an atomic mass of 1. | Helium has four nucleons so it has an atomic mass of 4. | Lithium has seven nucleons so it has an atomic mass of 7. | Beryllium has nine nucleons so it has an atomic mass of 9. |
Key Stage 3
Meaning

An element tile showing the mass number.
The relative atomic mass or mass number of an element is the number of nucleons (protons + neutrons) in an atom. The relative atomic mass in grams is also the mass of one mole or 6.02x1023 atoms of the element.
About Relative Atomic Mass
- The relative atomic mass in grams on the Periodic Table tells the mass of a mole of the element.
- A mole of the element is 6.02x1023 atoms of that element.
- Two atoms of the same element may have the same Atomic Number but a different Relative Atomic Mass depending on the number of neutrons in the nucleus. Elements with different mass numbers are called isotopes.
- The relative atomic mass is not affected by the number of electrons.
- Only the particles in the nucleus affect the relative atomic mass.
Examples
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has one nucleon so it has an atomic mass of 1 and 6.02x1023 (or 1 mole of) atoms of Hydrogen have a mass of 1g. | Helium has four nucleons so it has an atomic mass of 4 and 6.02x1023 (or 1 mole of) atoms of Helium have a mass of 4g. | Lithium has seven nucleons so it has an atomic mass of 7 and 6.02x1023 (or 1 mole of) atoms of Lithium have a mass of 7g. | Beryllium has nine nucleons so it has an atomic mass of 9 and 6.02x1023 (or 1 mole of) atoms of Beryllium have a mass of 9g. |