Difference between revisions of "Diffusion"
| Line 11: | Line 11: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | |[[File:Diffusion.png|center| | + | |[[File:Diffusion.png|center|600px]] |
|- | |- | ||
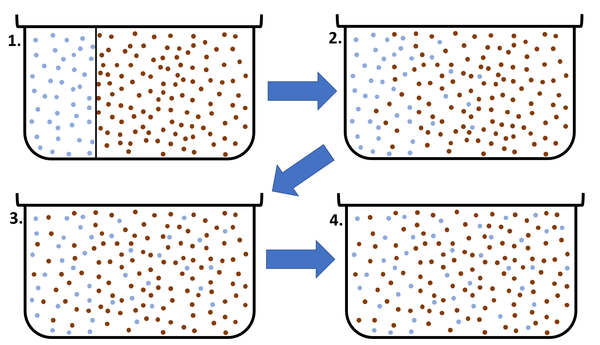
| − | | style="height:20px; width: | + | | style="height:20px; width:600px; text-align:center;" |This [[diagram]] shows a high [[concentration]] of blue [[particle]]s on the left separated from the red [[particle]]s by a barrier. When the barrier is removed the blue particles [[diffuse]] to the area of lower [[concentration]] on the right. [[Diffusion]] continues until all [[particle]]s are equally spread. |
|} | |} | ||
Revision as of 15:37, 22 September 2018
Key Stage 3
Meaning
Diffusion is when particles spread from a region of high concentration to a region of low concentration.
About Diffusion
- Diffusion can only happen in a fluid which means a liquid or a gas because the particles can move past each other.
- Diffusion cannot happen in a solid because the particles are held in fixed positions.
- In diffusion the particles always spread from a high concentration where there is lots of the substance, to a low concentration where there is less of the substance.
- Diffusion stops when all substances are spread out equally and there is no longer a concentration gradient.
| This diagram shows a high concentration of blue particles on the left separated from the red particles by a barrier. When the barrier is removed the blue particles diffuse to the area of lower concentration on the right. Diffusion continues until all particles are equally spread. |