Difference between revisions of "Group 7"
| Line 33: | Line 33: | ||
The [[reactivity]] decreases as you go down the group because: | The [[reactivity]] decreases as you go down the group because: | ||
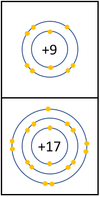
*The outer [[electron]]s are further away from the [[Atomic Nucleus|nucleus]] with each additional [[Electron Orbital|electron shell]] making the [[force]] of [[attract]]ion weaker. This makes it less able to gain an extra [[electron]]. | *The outer [[electron]]s are further away from the [[Atomic Nucleus|nucleus]] with each additional [[Electron Orbital|electron shell]] making the [[force]] of [[attract]]ion weaker. This makes it less able to gain an extra [[electron]]. | ||
| − | *Even though the [[Electrical Charge|charge]] of the [[Atomic Nucleus|nucleus]] increases the outer [[electron]]s are shielded from most of the [[Positive Charge|positive charge]] of the [[Atomic Nucleus|nucleus]] by [[electron]]s in the | + | *Even though the [[Electrical Charge|charge]] of the [[Atomic Nucleus|nucleus]] increases the outer [[electron]]s are shielded from most of the [[Positive Charge|positive charge]] of the [[Atomic Nucleus|nucleus]] by [[electron]]s in the inner shells. |
|} | |} | ||
Revision as of 10:44, 6 April 2019
Contents
Key Stage 4
Meaning
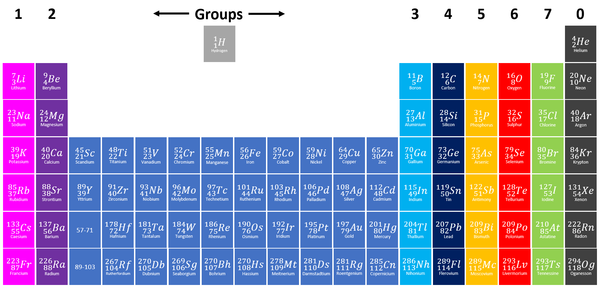
Group 7 elements, also known as Halogens on the Periodic Table are the elements which have 7 electrons in their outer shell.
| Group 7 elements are shown in green at the right of the Periodic Table. |
About the Halogens
- The Halogens have similar chemical properties because they all have 7 electrons on their outer shell.
- Halogens all produce ions with a -1 relative charge because they gain an electron in chemical reactions.
The Halogens in order from most reactive to least reactive are:
Chemical Properties
- The reactivity of Halogens decreases as you go down the Periodic Table.
- Halogens all react strongly as bleaching agents.
- Halogens all produce acids when combined with Hydrogen.
- Halogens are toxic to bacteria and are used in disinfectants.
| In a chemical reaction an extra electron is added to the outer shell.
The reactivity decreases as you go down the group because:
|
Physical Properties
The physical properties of Halogens changes significantly as you go down the Periodic Table:
- Fluorine - A yellow gas at room temperature.
- Chlorine - A green gas at room temperature.
- Bromine - A brown liquid at room temperature.
- Iodine - A purple solid at room temperature.
- Astatine -A dark purple solid at room temperature.
- The density, melting point and boiling point all increase as you go down the Periodic Table.