Difference between revisions of "Group 7"
(→Chemical Properties) |
|||
| Line 25: | Line 25: | ||
: '''Halogens''' all produce [[acid]]s when combined with [[Hydrogen]]. | : '''Halogens''' all produce [[acid]]s when combined with [[Hydrogen]]. | ||
: '''Halogens''' are [[toxic]] to [[bacteria]] and are used in [[disinfectant]]s. | : '''Halogens''' are [[toxic]] to [[bacteria]] and are used in [[disinfectant]]s. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:Group7ElectronShells.png|center|100px]] | ||
| + | | style="height:20px; width:500px; text-align:left;" |In a [[Chemical Reaction|chemical reaction]] an extra [[electron]] is added to the [[Outer Shell|outer shell]]. | ||
| + | The [[reactivity]] decreases as you go down the group because: | ||
| + | *The outer [[electron]]s are further away from the [[Atomic Nucleus|nucleus]] with each additional [[Electron Orbital|electron shell]] making the [[force]] of [[attract]]ion weaker. This makes it less able to gain an extra [[electron]]. | ||
| + | *Even though the [[charge]] of the [[Atomic Nucleus|nucleus]] increases the outer [[electron]]s are shielded from most of the [[Positive Charge|positive charge]] of the [[Atomic Nucleus|nucleus]] by [[electron]]s in the [[Inner Shell|inner shells]]. | ||
| + | |} | ||
===Physical Properties=== | ===Physical Properties=== | ||
The [[Physical Property|physical properties]] of '''Halogens''' changes significantly as you go down the [[Periodic Table]]: | The [[Physical Property|physical properties]] of '''Halogens''' changes significantly as you go down the [[Periodic Table]]: | ||
Revision as of 18:12, 26 November 2018
Contents
Key Stage 4
Meaning
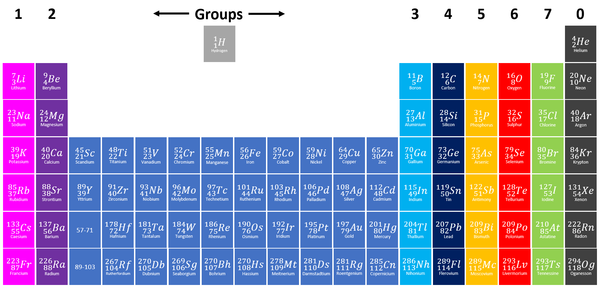
Group 7 elements, also known as Halogens on the Periodic Table are the elements which have 7 electrons in their outer shell.
| Group 7 elements are shown in green at the right of the Periodic Table. |
About the Halogens
- The Halogens have similar chemical properties because they all have 7 electrons on their outer shell.
- Halogens all produce ions with a -1 relative charge because they gain an electron in chemical reactions.
The Halogens in order from most reactive to least reactive are:
Chemical Properties
- The reactivity of Halogens decreases as you go down the Periodic Table.
- Halogens all react strongly as bleaching agents.
- Halogens all produce acids when combined with Hydrogen.
- Halogens are toxic to bacteria and are used in disinfectants.
| In a chemical reaction an extra electron is added to the outer shell.
The reactivity decreases as you go down the group because:
|
Physical Properties
The physical properties of Halogens changes significantly as you go down the Periodic Table:
- Fluorine - A yellow gas at room temperature.
- Chlorine - A green gas at room temperature.
- Bromine - A brown liquid at room temperature.
- Iodine - A purple solid at room temperature.
- Astatine -A dark purple solid at room temperature.
- The density, melting point and boiling point all increase as you go down the Periodic Table.