Difference between revisions of "Condensing"
| Line 43: | Line 43: | ||
|- | |- | ||
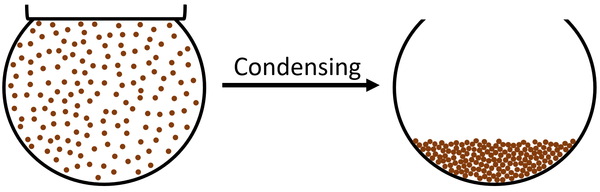
| style="height:20px; width:600px; text-align:left;" |The [[particle]]s in the [[gas]] slow down until they are slow enough that [[force]]s between the [[particle]]s start to hold them together. The [[particle]]s end up touching but can still move past each other which makes the [[State of Matter|state]] a [[liquid]]. | | style="height:20px; width:600px; text-align:left;" |The [[particle]]s in the [[gas]] slow down until they are slow enough that [[force]]s between the [[particle]]s start to hold them together. The [[particle]]s end up touching but can still move past each other which makes the [[State of Matter|state]] a [[liquid]]. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Condensing]] is an [[exothermic]] [[Physical Change|physical change]] in which a [[gas]] turns into a [[liquid]]. | ||
| + | |||
| + | ===About Condensing=== | ||
| + | : [[Condensing]] happens when the [[particle]]s in a [[gas]] form [[bond]]s holding them together as they lose [[Potential Energy|potential energy]]. | ||
| + | : The [[temperature]] at which a [[substance]] '''condenses''' is the same as the [[temperature]] at which it [[boiling|boils]] so it is the [[Boiling Point|boiling point]]. | ||
| + | : [[Condensing]] is an [[exothermic]] process, which means it [[emit]]s [[energy]] when it takes place. | ||
| + | : [[Condensing]] is a [[Physical Change|physical change]], which means it is [[Reversible Changes|reversible]] and does not produce new [[chemical]]s. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |[[File:CondensingGraph.png|center|500px]] | ||
| + | |- | ||
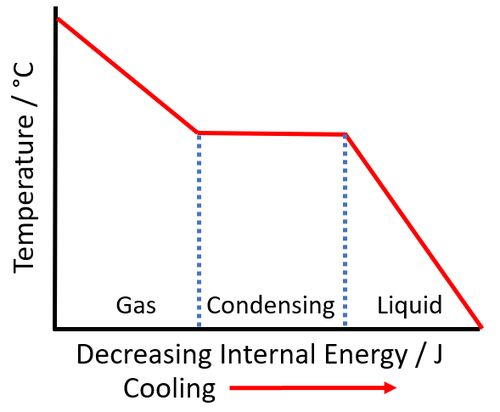
| + | | style="height:20px; width:500px; text-align:left;" |As [[energy]] is removed from the [[liquid]] it '''freezes''' and the [[temperature]] of the [[substance]] stays the same. | ||
|} | |} | ||
Revision as of 11:52, 27 December 2018
Contents
Key Stage 2
Meaning
Condensing is when a gas turns into a liquid.
- Noun: Condensation
- Verb: To condense
- Present Participle: Condensing
| A gas will condense to become a liquid. |
About Condensing
- Cooling a gas will cause it to condense into a liquid.
- Condensing is a reversible process. When a gas condenses into a liquid you can evaporate that liquid back into a gas.
- You may have seen water condensing.
| Water Vapour in the air condenses into liquid water onto a cold bottle. | The Steam coming out of kettle condenses into a cloud of liquid water droplets. |
Key Stage 3
Meaning
Condensing is an exothermic process in which a gas turns into a liquid.
About Condensing
- Condensing is a reversible process. When a gas condenses into a liquid you can evaporate that liquid back into a gas.
- Cooling a gas will cause it to condense into a liquid.
| The particles in the gas slow down until they are slow enough that forces between the particles start to hold them together. The particles end up touching but can still move past each other which makes the state a liquid. |
Key Stage 4
Meaning
Condensing is an exothermic physical change in which a gas turns into a liquid.
About Condensing
- Condensing happens when the particles in a gas form bonds holding them together as they lose potential energy.
- The temperature at which a substance condenses is the same as the temperature at which it boils so it is the boiling point.
- Condensing is an exothermic process, which means it emits energy when it takes place.
- Condensing is a physical change, which means it is reversible and does not produce new chemicals.
| As energy is removed from the liquid it freezes and the temperature of the substance stays the same. |