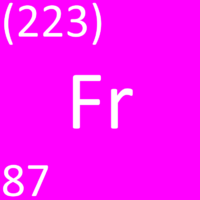Difference between revisions of "Francium"
(Created page with "==Key Stage 2== ===Meaning=== Francium is a metal. ==Key Stage 3== ===Meaning=== right|300px|thumb|The [[Chemical Symbol|chemical symbol fo...") |
|||
| (11 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
| − | [[File: | + | [[File:FranciumSymbol1.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Francium]].]] |
| − | [[Francium]] is a [[Group 1]] [[element]] with an [[Atomic Number|atomic number]] of 87. | + | [[Francium]] is a [[Group 1]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 87. |
===About Francium=== | ===About Francium=== | ||
| − | : [[Francium]] has the [[Chemical | + | ====Molecular Structure==== |
| + | : [[Francium]] has the [[Chemical Symbol|chemical symbol]] [[Francium|Fr]]. | ||
| + | : [[Francium]] [[atom]]s join together in large numbers to form a giant [[metal]] [[molecule]]. | ||
| + | ====Atomic Structure==== | ||
: [[Francium]] as 87 [[proton]]s and 136 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 87 and an [[Relative Atomic Mass|atomic mass]] of 223. | : [[Francium]] as 87 [[proton]]s and 136 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 87 and an [[Relative Atomic Mass|atomic mass]] of 223. | ||
| + | : An [[atom]] of [[Francium]] has only 1 [[electron]] in its [[Outer Shell|outer shell]]. | ||
| + | ====Properties==== | ||
: [[Francium]] is the most [[Reactivity|reactive]] [[Alkali Metal|alkali metal]]. | : [[Francium]] is the most [[Reactivity|reactive]] [[Alkali Metal|alkali metal]]. | ||
| − | : [[Francium]] | + | : [[Francium]] does not exist in nature but has been created by [[scientist]]s. |
| − | : [[Francium]] [[Chemical Reaction| | + | : [[Francium]] would [[Chemical Reaction|react]] strongly with [[water]] to produce [[Hydrogen]] [[gas]] and Francium Hydroxide. |
| − | + | ||
| − | |||
==Key Stage 4== | ==Key Stage 4== | ||
| + | [[File:FrKS4.PNG|right|200px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Francium]].]] | ||
===Meaning=== | ===Meaning=== | ||
| − | [[Francium]] is a [[Group 1]] [[element]] with 87 [[proton]]s in the [[Atomic Nucleus|nucleus]]. | + | [[Francium]] is a [[Group 1]] [[element]], on the [[Periodic Table]], with 87 [[proton]]s in the [[Atomic Nucleus|nucleus]]. |
===About Francium=== | ===About Francium=== | ||
| − | : [[Francium]] has the [[Chemical | + | ====Molecular Structure==== |
| + | : [[Francium]] has the [[Chemical Symbol|chemical symbol]] [[Francium|Fr]]. | ||
| + | : [[Francium]] [[atom]]s join together in a [[Giant Metallic Structure|giant metallic structure]]. | ||
| + | ====Atomic Structure==== | ||
: The most [[Stable Isotope|stable isotope]] of [[Francium]] has 136 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 223. | : The most [[Stable Isotope|stable isotope]] of [[Francium]] has 136 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 223. | ||
| + | : An [[atom]] of [[Francium]] has only 1 [[electron]] in its [[Outer Shell|outer shell]]. | ||
| + | : [[Francium]] [[ion]]s have lost an [[electron]] to become [[Positive Charge|positively charged]]. | ||
| + | ====Properties==== | ||
: [[Francium]] is the most [[Reactivity|reactive]] [[Alkali Metal|alkali metal]]. | : [[Francium]] is the most [[Reactivity|reactive]] [[Alkali Metal|alkali metal]]. | ||
| − | : [[Francium]] | + | : [[Francium]] does not exist in nature but has been produced in small quantities in [[Particle Accelerator|particle accelerators]]. |
| − | : [[Francium]] [[Chemical Reaction| | + | : [[Francium]] would [[Chemical Reaction|react]] strongly with [[water]] to produce [[Hydrogen]] [[gas]] and Francium Hydroxide. |
| − | |||
| − | |||
Latest revision as of 14:42, 5 March 2020
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Francium is a Group 1 element, on the Periodic Table, with an atomic number of 87.
About Francium
Molecular Structure
- Francium has the chemical symbol Fr.
- Francium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Francium as 87 protons and 136 neutrons in its nucleus giving it an Atomic Number of 87 and an atomic mass of 223.
- An atom of Francium has only 1 electron in its outer shell.
Properties
- Francium is the most reactive alkali metal.
- Francium does not exist in nature but has been created by scientists.
- Francium would react strongly with water to produce Hydrogen gas and Francium Hydroxide.
Key Stage 4
Meaning
Francium is a Group 1 element, on the Periodic Table, with 87 protons in the nucleus.
About Francium
Molecular Structure
- Francium has the chemical symbol Fr.
- Francium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Francium has 136 neutrons in its nucleus giving it an atomic mass of 223.
- An atom of Francium has only 1 electron in its outer shell.
- Francium ions have lost an electron to become positively charged.
Properties
- Francium is the most reactive alkali metal.
- Francium does not exist in nature but has been produced in small quantities in particle accelerators.
- Francium would react strongly with water to produce Hydrogen gas and Francium Hydroxide.