Key Stage 3
Meaning
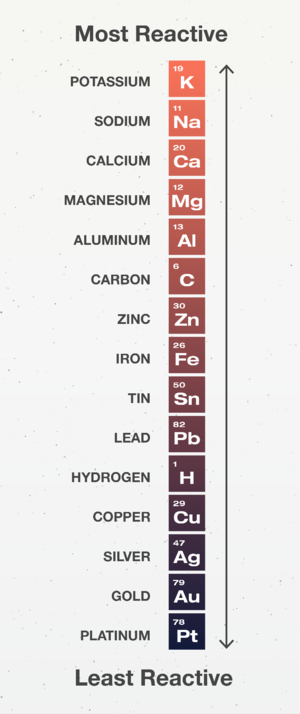
The Reactivity Series is a list of elements in order of their reactivity.
About The Reactivity Series
- The Reactivity Series is used to predict the outcome of Displacement Reactions. Elements higher on the reactivity series will displace those lower down on the reactivity series.
- Carbon and Hydrogen are important in the Reactivity Series as they can indicate how a metal can be extracted from its ore.
- Elements below Hydrogen are found Native which means the metal element can be found not as part of a compound.
- Elements above Hydrogen but below Carbon are found in metal compounds so they need to be extracted by using Carbon to displace the metal from the compound.
- Elements above Carbon are found in metal compounds but they cannot be extracted with Carbon because Carbon is less reactive than those metals so we use electrolysis to extract them.