Difference between revisions of "Isotope"
(Created page with "==Key Stage 4== ===Meaning=== Isotopes are atoms with the same number of protons (the same element) but a different number of neutrons. ===About Isotopes=...") |
|||
| Line 6: | Line 6: | ||
: Each [[element]] has many [[isotope]]s but some are [[Stable Isotope|stable]] and others are [[Unstable Isotope|unstable]] so they [[Radioactive Decay|decay]] quickly into other [[isotope]]s or a different [[element]]. | : Each [[element]] has many [[isotope]]s but some are [[Stable Isotope|stable]] and others are [[Unstable Isotope|unstable]] so they [[Radioactive Decay|decay]] quickly into other [[isotope]]s or a different [[element]]. | ||
: Different [[isotope]]s of the same [[element]] have the same [[Atomic Number|atomic number]] but different [[Relative Atomic Mass|atomic mass]] due to the different numbers of [[neutron]]s. | : Different [[isotope]]s of the same [[element]] have the same [[Atomic Number|atomic number]] but different [[Relative Atomic Mass|atomic mass]] due to the different numbers of [[neutron]]s. | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen-1''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen-2''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Lithium-7''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Lithium-6''' | ||
| + | |- | ||
| + | |[[File:Hydrogen.png|center|150px]] | ||
| + | |[[File:Deuterium.png|center|150px]] | ||
| + | |[[File:Lithium.png|center|200px]] | ||
| + | |[[File:Lithium6.png|center|200px]] | ||
| + | |- | ||
| + | |[[File:HydrogenSymbol.png|center|200px]] | ||
| + | |[[File:DeuteriumSymbol.png|center|200px]] | ||
| + | |[[File:LithiumSymbol.png|center|200px]] | ||
| + | |[[File:Lithium6Symbol.png|center|200px]] | ||
| + | |- | ||
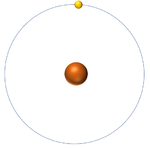
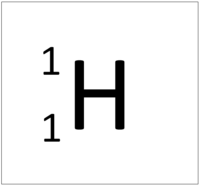
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] always has 1 [[proton]] but in this case has no [[neutron]]s. | ||
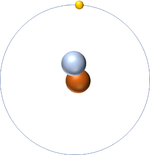
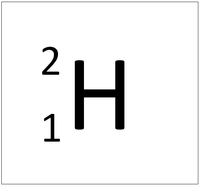
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] always has 1 [[proton]] but in this case also has a [[neutron]]. This [[isotope]] of [[Hydrogen]] is known as [[Deuterium]]. | ||
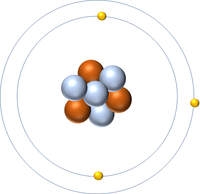
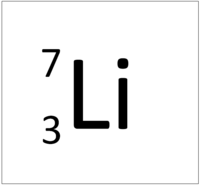
| + | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] always has 3 [[proton]]s but in this case has 4 [[neutron]]s. | ||
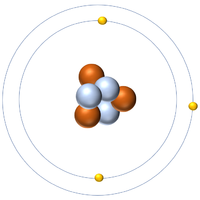
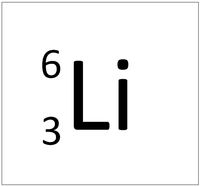
| + | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] always has 3 [[proton]]s but in this case has 3 [[neutron]]s. | ||
| + | |} | ||
Revision as of 00:16, 26 November 2018
Key Stage 4
Meaning
Isotopes are atoms with the same number of protons (the same element) but a different number of neutrons.
About Isotopes
- Each element has many isotopes but some are stable and others are unstable so they decay quickly into other isotopes or a different element.
- Different isotopes of the same element have the same atomic number but different atomic mass due to the different numbers of neutrons.
| Hydrogen-1 | Hydrogen-2 | Lithium-7 | Lithium-6 |
| Hydrogen always has 1 proton but in this case has no neutrons. | Hydrogen always has 1 proton but in this case also has a neutron. This isotope of Hydrogen is known as Deuterium. | Lithium always has 3 protons but in this case has 4 neutrons. | Lithium always has 3 protons but in this case has 3 neutrons. |