Difference between revisions of "Group (Chemistry)"
(Created page with "==Key Stage 3== ===Meaning=== A Group is a column on the Periodic Table with elements with the same number of electrons on the Outer Shell. ===About Group...") |
|||
| Line 4: | Line 4: | ||
===About Groups=== | ===About Groups=== | ||
| − | : The [[element]]s are arranged [[group]]s of similar [[Chemical | + | : The [[element]]s are arranged [[group]]s of similar [[Chemical Property|chemical properties]]. |
| − | : [[Element]]s have similar [[Chemical | + | : [[Element]]s have similar [[Chemical Property|chemical properties]] when they have the same number of [[electron]]s in the [[Outer Shell]]. |
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 09:24, 1 October 2018
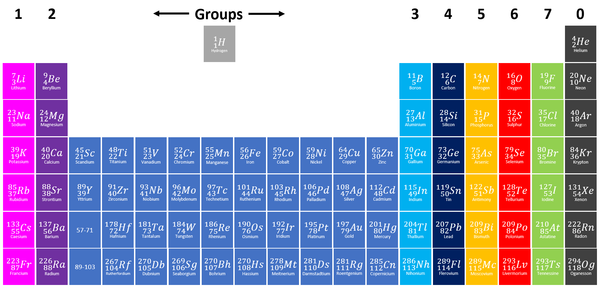
Key Stage 3
Meaning
A Group is a column on the Periodic Table with elements with the same number of electrons on the Outer Shell.
About Groups
- The elements are arranged groups of similar chemical properties.
- Elements have similar chemical properties when they have the same number of electrons in the Outer Shell.
Trends within groups
The chemical properties of elements within a [[group] are similar. However, the reactivity within a group changes as you move up or down the periods.
- Group 1: The Alkali Metals all react strongly with water. The reactivity increases as you go down the group.
- Group 2: The Alkali Earth Metals all react strongly with steam and acids. The reactivity increases as you go down the group.
- Group 7: The Halogens all act as bleaching agents and kill bacteria. The reactivity decreases as you go down the group.
- Group 0: The Noble Gases are all inert (unreactive).
The physical properties of elements within a group are similar. However, the property changes gradually as you move down the group.