Difference between revisions of "Pure"
(→About Purity) |
(→Key Stage 4) |
||
| Line 39: | Line 39: | ||
: '''Purity''' is important for ensuring that when [[Chemical Reaction|reaction]]s take place there are no unwanted [[product]]s caused by the '''impurities'''. | : '''Purity''' is important for ensuring that when [[Chemical Reaction|reaction]]s take place there are no unwanted [[product]]s caused by the '''impurities'''. | ||
: '''Impurities''' can change the [[property|properties]] of a [[substance]] such as its [[Melting Point|melting point]], [[Electrical Conductivity|electrical conductivity]] or [[Strength (Property)|strength]]. This means '''purity''' is essential when using [[substance]]s for certain [[application]]s where those [[property|properties]] important. | : '''Impurities''' can change the [[property|properties]] of a [[substance]] such as its [[Melting Point|melting point]], [[Electrical Conductivity|electrical conductivity]] or [[Strength (Property)|strength]]. This means '''purity''' is essential when using [[substance]]s for certain [[application]]s where those [[property|properties]] important. | ||
| − | + | ||
'''Purity''' may refer to: | '''Purity''' may refer to: | ||
*A '''pure''' [[element]] - A [[substance]] containing only one type of [[atom]]. | *A '''pure''' [[element]] - A [[substance]] containing only one type of [[atom]]. | ||
*A '''pure''' [[compound]] - A [[substance]] containing only one [[chemical]] [[compound]]. | *A '''pure''' [[compound]] - A [[substance]] containing only one [[chemical]] [[compound]]. | ||
| + | |||
| + | ===Detecting Purity=== | ||
| + | : The '''purity''' of a sample can be checked by looking at its [[property|properties]] such as the [[Melting Point|melting point]] and [[Boiling Point|boiling point]]. If these are spread over a range of [[temperature]]s or different from the known values for the [[pure]] [[substance]] then the sample is not [[pure]]. | ||
| + | : To test if a sample of [[Water]] is [[pure]] it can be [[melting|melted]] from [[Ice]] and [[boiling|boiled]] from [[liquid]] [[Water]]. If the [[Melting Point|melting point]] is exactly 0°C and the [[Boiling Point|boiling point]] is exactly 100°C, then the [[Water]] is [[pure]]. | ||
Revision as of 11:17, 20 January 2019
Contents
Key Stage 3
Meaning
A pure substance is one that contains only one type of chemical.
About Pure Substances
- The opposite of a pure substance would be a mixture of substances.
- A substance can be pure because it is made of only one element or it can be pure because it is made of only one compound.
- The word pure is often used in food adverts to mean there is only one ingredient, eg pure orange juice contains only oranges, but most people confuse it for meaning something that is healthy, natural or good. Be careful not to confuse this for the real meaning of pure.
Examples
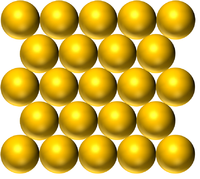
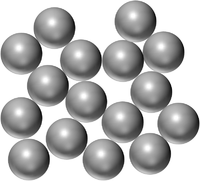
| Pure Gold is a solid made of only Gold atoms. | Pure Mercury is a Liquid made of only Mercury atoms. | Pure Oxygen is a gas made of only Oxygen atoms. |
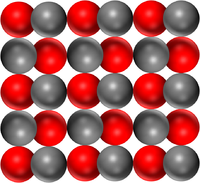
| Pure Magnesium Oxide is a solid made of only the compound Magnesium Oxide | Pure water is a liquid made of only the compound H2O. | Pure Carbon Dioxide is a gas made of only Carbon Dioxide molecules. |
Key Stage 4
Meaning
A pure substance is one that contains only one type of chemical.
About Purity
- Pure does NOT mean good, healthy or natural.
- Purity is important for ensuring that when reactions take place there are no unwanted products caused by the impurities.
- Impurities can change the properties of a substance such as its melting point, electrical conductivity or strength. This means purity is essential when using substances for certain applications where those properties important.
Purity may refer to:
- A pure element - A substance containing only one type of atom.
- A pure compound - A substance containing only one chemical compound.
Detecting Purity
- The purity of a sample can be checked by looking at its properties such as the melting point and boiling point. If these are spread over a range of temperatures or different from the known values for the pure substance then the sample is not pure.
- To test if a sample of Water is pure it can be melted from Ice and boiled from liquid Water. If the melting point is exactly 0°C and the boiling point is exactly 100°C, then the Water is pure.