Difference between revisions of "Reactivity Series"
| Line 6: | Line 6: | ||
===About The Reactivity Series=== | ===About The Reactivity Series=== | ||
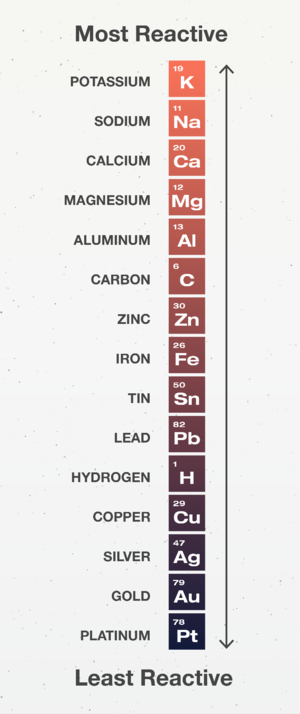
: The '''Reactivity Series''' is used to predict the outcome of [[Displacement Reaction]]s. [[Element]]s higher on the '''reactivity series''' will [[Displacement Reaction|displace]] those lower down on the '''reactivity series'''. | : The '''Reactivity Series''' is used to predict the outcome of [[Displacement Reaction]]s. [[Element]]s higher on the '''reactivity series''' will [[Displacement Reaction|displace]] those lower down on the '''reactivity series'''. | ||
| − | : [[Carbon]] and [[Hydrogen]] are important in the [[Reactivity Series]] as they can indicate how a [[metal]] can be [[ | + | : [[Carbon]] and [[Hydrogen]] are important in the [[Reactivity Series]] as they can indicate how a [[metal]] can be [[Extraction of Metals|extracted]] from its [[ore]]. |
: [[Element]]s below [[Hydrogen]] are found [[Native]] which means the [[metal]] [[element]] can be found not as part of a [[compound]]. | : [[Element]]s below [[Hydrogen]] are found [[Native]] which means the [[metal]] [[element]] can be found not as part of a [[compound]]. | ||
| − | : [[Element]]s above [[Hydrogen]] but below [[Carbon]] are found in [[metal]] [[compound]]s so they need to be [[ | + | : [[Element]]s above [[Hydrogen]] but below [[Carbon]] are found in [[metal]] [[compound]]s so they need to be [[Extraction of Metals|extracted]] by using [[Carbon]] to [[Displacement Reaction|displace]] the [[metal]] from the [[compound]]. |
| − | : [[Element]]s above [[Carbon]] are found in [[metal]] [[compound]]s but they cannot be [[ | + | : [[Element]]s above [[Carbon]] are found in [[metal]] [[compound]]s but they cannot be [[Extraction of Metals|extracted]] with [[Carbon]] because [[Carbon]] is less [[Reactivity|reactive]] than those [[metal]]s so we use [[electrolysis]] to [[Extraction of Metals|extract]] them. |
Revision as of 15:20, 1 October 2018
Key Stage 3
Meaning
The Reactivity Series is a list of elements in order of their reactivity.
About The Reactivity Series
- The Reactivity Series is used to predict the outcome of Displacement Reactions. Elements higher on the reactivity series will displace those lower down on the reactivity series.
- Carbon and Hydrogen are important in the Reactivity Series as they can indicate how a metal can be extracted from its ore.
- Elements below Hydrogen are found Native which means the metal element can be found not as part of a compound.
- Elements above Hydrogen but below Carbon are found in metal compounds so they need to be extracted by using Carbon to displace the metal from the compound.
- Elements above Carbon are found in metal compounds but they cannot be extracted with Carbon because Carbon is less reactive than those metals so we use electrolysis to extract them.