Difference between revisions of "Tritium"
(Created page with "==Key Stage 4== ===Meaning=== right|300px|thumb|The [[Chemical Symbol|chemical symbol for Deuterium.]] Tritium is an isotope of Hydrog...") |
|||
| Line 8: | Line 8: | ||
{| class="wikitable" | {| class="wikitable" | ||
| − | |[[File: | + | |[[File:Tritium.png|center|200px]] |
| − | |[[File: | + | |[[File:TritiumSymbol.png|center|200px]] |
|- | |- | ||
| style="height:20px; width:200px; text-align:center;" colspan = "2"|[[Hydrogen]] always has 1 [[proton]] but in this [[isotope]] there is 1 [[neutron]]. This [[isotope]] of [[Hydrogen]] is known as [[Deuterium]]. | | style="height:20px; width:200px; text-align:center;" colspan = "2"|[[Hydrogen]] always has 1 [[proton]] but in this [[isotope]] there is 1 [[neutron]]. This [[isotope]] of [[Hydrogen]] is known as [[Deuterium]]. | ||
|} | |} | ||
Revision as of 11:52, 31 March 2019
Key Stage 4
Meaning
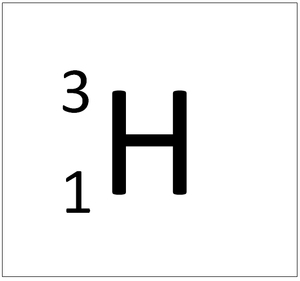
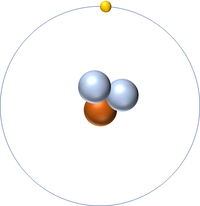
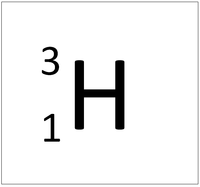
Tritium is an isotope of Hydrogen containing two neutrons.
About Tritium
- Tritium has all the same chemical properties of Hydrogen.
- Tritium is an unstable isotope with a half life of around 12.3 years.
| Hydrogen always has 1 proton but in this isotope there is 1 neutron. This isotope of Hydrogen is known as Deuterium. | |