Difference between revisions of "Ethane"
(Created page with "Ethane is a gaseous (at room temperature) chemical compound with formula C<sub>2</sub>H<sub>6</sub>.") |
|||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Ethane]] is a [[gas]]eous (at [[Room Temperature|room temperature]]) chemical [[ | + | ==Key Stage 3== |
| + | ===Meaning=== | ||
| + | [[Ethane]] is a [[gas]]eous (at [[Room Temperature|room temperature]]) [[hydrocarbon]] with [[Chemical Formula|chemical formula]] C<sub>2</sub>H<sub>6</sub>. | ||
| + | |||
| + | ===About Ethane=== | ||
| + | : [[Ethane]] is [[hydrocarbon]] because it contains only [[Hydrogen]] and [[Carbon]] [[atom]]s. | ||
| + | : [[Ethane]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | ||
| + | : [[Ethane]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Ethane]] is a [[gas]]eous (at [[Room Temperature|room temperature]]) [[alkane]] with [[Chemical Formula|chemical formula]] C<sub>2</sub>H<sub>6</sub>. | ||
| + | |||
| + | ===About Ethane=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:125px; text-align:center;" |[[Chemical Formula]] (C<sub>n</sub>H<sub>2n+2</sub>) | ||
| + | | style="height:20px; width:125px; text-align:center;" |[[Structural Formula]] | ||
| + | | style="height:20px; width:125px; text-align:center;" |[[Structural Diagram]] | ||
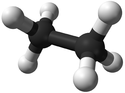
| + | | style="height:20px; width:125px; text-align:center;" |[[Ball and Stick Model]] | ||
| + | |- | ||
| + | | style="height:20px; width:125px; text-align:center;" |C<sub>2</sub>H<sub>6</sub> | ||
| + | | style="height:20px; width:125px; text-align:center;" |CH<sub>3</sub>CH<sub>3</sub> | ||
| + | |[[File:StructuralDiagramEthane.png|center|125px]] | ||
| + | |[[File:BallandStickEthane.png|center|125px]] | ||
| + | |} | ||
| + | |||
| + | : [[Ethane]] is [[hydrocarbon]] because it contains only [[Hydrogen]] and [[Carbon]] [[atom]]s. | ||
| + | : [[Ethane]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | ||
| + | : [[Ethane]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | : 2C<sub>2</sub>H<sub>6</sub> + 7O<sub>2</sub> → 4CO<sub>2</sub> + 6H<sub>2</sub>O | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Ethane, page 188, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Ethane, page 195, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Ethane, page 220, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Ethane, pages 99, 228-9, 238, 254, 256, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120207/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120207&linkCode=as2&tag=nrjc-21&linkId=22455ff53961978667722edaa64c0be5 ''Ethane gas, pages 137, 139, GCSE Biology, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948120/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948120&linkCode=as2&tag=nrjc-21&linkId=dedef775c6a43dbb0a609441525adac0 ''Ethane, page 219, GCSE Biology, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945660/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945660&linkCode=as2&tag=nrjc-21&linkId=83aa4500ad7759e7f401a1c5ba5df758 ''Ethane, page 51, Gateway GCSE Biology; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 15:13, 6 December 2019
Contents
Key Stage 3
Meaning
Ethane is a gaseous (at room temperature) hydrocarbon with chemical formula C2H6.
About Ethane
- Ethane is hydrocarbon because it contains only Hydrogen and Carbon atoms.
- Ethane can be oxidised to produce Carbon Dioxide and Water.
- Ethane + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Ethane is a gaseous (at room temperature) alkane with chemical formula C2H6.
About Ethane
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C2H6 | CH3CH3 |
- Ethane is hydrocarbon because it contains only Hydrogen and Carbon atoms.
- Ethane can be oxidised to produce Carbon Dioxide and Water.
- Ethane + Oxygen → Carbon Dioxide + Water
- 2C2H6 + 7O2 → 4CO2 + 6H2O
References
AQA
- Ethane, page 188, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Ethane, page 195, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Ethane, page 220, GCSE Chemistry, CGP, AQA
- Ethane, pages 99, 228-9, 238, 254, 256, GCSE Chemistry; Student Book, Collins, AQA
Edexcel
- Ethane gas, pages 137, 139, GCSE Biology, Pearson, Edexcel
- Ethane, page 219, GCSE Biology, CGP, Edexcel