Key Stage 4
Meaning
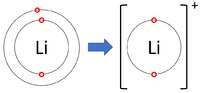
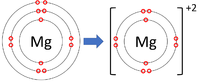
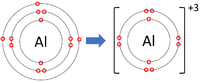
Positive ions are elements which have lost one or more electrons to become positively charged.
About Positive Ions
- In chemical reactions between metals and non-metals the metal elements form positive ions.
- Hydrogen forms positive ions in some compounds and it is these H+ ions which can make solutions acidic.
- Positive ions are attracted to negative ions and to the negative electrode (cathode) during electrolysis.
Examples
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |