Key Stage 3
Meaning
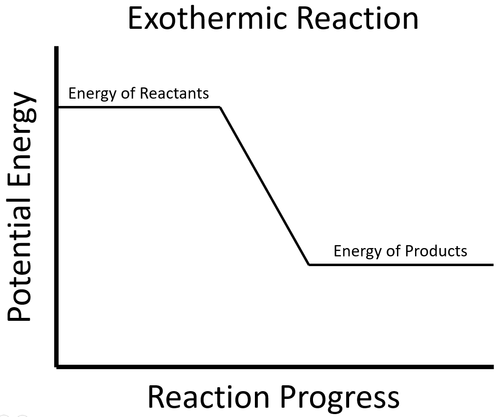
An exothermic process is one that gives out energy. This usually causes surroundings to increase in temperature.
About Exothermic Processes
- Most chemical reactions are exothermic with means they release energy to the environment and this is observed by an increase in temperature.
| The energy stored in the reactants is released in the chemical reaction making the material increase in temperature. The products now have less energy than the reactants. |
- Freezing, Condensing and Depositing are exothermic changes because they release energy when they happen. The material has less after they have happened. However, there is usually no increase in temperature because the material is usually cooled to change state.