Contents
Key Stage 3
Meaning
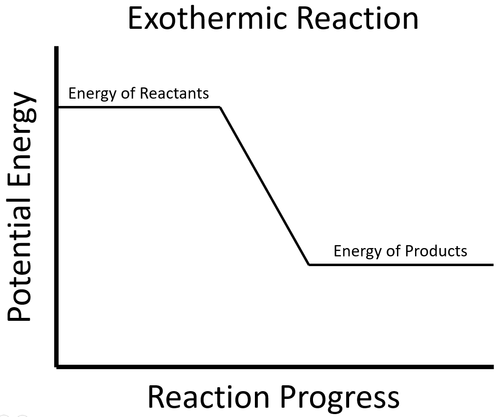
An exothermic process is one that gives out energy. This usually causes surroundings to increase in temperature.
About Exothermic Processes
- Most chemical reactions are exothermic with means they release energy to the environment and this is observed by an increase in temperature.
| The energy stored in the reactants is released in the chemical reaction making the material increase in temperature. The products now have less energy than the reactants. |
- Freezing, Condensing and Depositing are exothermic changes because they release energy when they happen. The material has less after they have happened. However, there is usually no increase in temperature because the material is usually cooled to change state.
Key Stage 4
Meaning
An exothermic process is one that gives out energy. This usually causes surroundings to increase in temperature.
About Exothermic Processes
Foundation
- Exothermic reactions usually require energy to begin. This is called the activation energy.
- In an exothermic process the potential energy stored in the products is less than the potential energy stored in the reactants.
| To start the reaction an activation energy is needed. This is usually achieved by initially heating the reactants. Once the reaction starts the energy stored in the reactants is released in the chemical reaction making the material increase in temperature. The products now have less energy than the reactants. |
Higher
- In a chemical reaction energy is needed to break the chemical bonds holding the atoms together.
- In an exothermic reaction energy is then released as atoms form new chemical bonds.
- In an exothermic reaction more energy is released in the formation of new bonds in the product than is taken in in the breaking of bonds in the reactants. So there is a net output of energy.
Examples
Higher
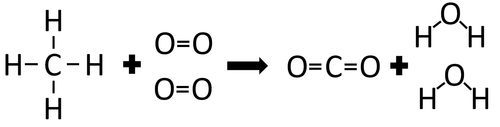
Example 1
| In the reaction between Methane and Oxygen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
| Bond | Energy in kJ/mol |
| C-H | 413 |
| O=O | 498 |
| O-H | 464 |
| C=O | 799 |
There are 4 C-H bonds and 2 O=O bonds.
4 x 413 + 2 x 498 = 2648kJ
Therefore 2648kJ/mol are needed to break the bonds in the reactants.
There are 2 C=O bonds and 4 O-H bonds.
2 x 799 + 4 x 464 = 3454kJ
Therefore 3454kJ/mol is released when the bonds in the products form.
Once the reaction is complete 806kJ will be released.
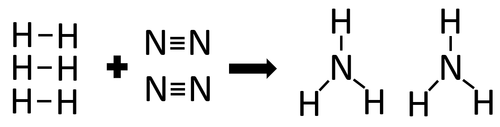
Example 2
| In the reaction between Hydrogen and Nitrogen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
| Bond | Energy in kJ/mol |
| H-H | 436 |
| N≡N | 941 |
| N-H | 391 |
There are 3 H-H bonds and 1 N≡N bonds.
3 x 436 + 1 x 941 = 2249kJ
Therefore 2249kJ/mol are needed to break the bonds in the reactants.
There are 6 N-H bonds.
6 x 391 = 2346kJ
Therefore 2346kJ/mol is released when the bonds in the products form.
Once the reaction is complete 97kJ will be released.