Difference between revisions of "Polymerisation"
(→Examples) |
|||
| Line 13: | Line 13: | ||
|- | |- | ||
|[[File:StructuralDiagramEthene.png|center|125px]] | |[[File:StructuralDiagramEthene.png|center|125px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:PolytheneFormula.png|center|200px]] | |[[File:PolytheneFormula.png|center|200px]] | ||
|- | |- | ||
| Line 24: | Line 24: | ||
|- | |- | ||
|[[File:StructuralDiagramTetrafluoroethene.png|center|125px]] | |[[File:StructuralDiagramTetrafluoroethene.png|center|125px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:PolyTetraFluoroEtheneFormula.png|center|200px]] | |[[File:PolyTetraFluoroEtheneFormula.png|center|200px]] | ||
|- | |- | ||
| Line 35: | Line 35: | ||
|- | |- | ||
|[[File:StructuralDiagramPropene.png|center|200px]] | |[[File:StructuralDiagramPropene.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:PolyPropeneFormula.png|center|200px]] | |[[File:PolyPropeneFormula.png|center|200px]] | ||
|- | |- | ||
| Line 46: | Line 46: | ||
|- | |- | ||
|[[File:StructuralDiagramEthandioateEthandiol.png|center|200px]] | |[[File:StructuralDiagramEthandioateEthandiol.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:StructuralDiagramPolyester.png|center|200px]] | |[[File:StructuralDiagramPolyester.png|center|200px]] | ||
|- | |- | ||
| Line 57: | Line 57: | ||
|- | |- | ||
|[[File:StructuralDiagramGlucose.png|center|200px]] | |[[File:StructuralDiagramGlucose.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:StructuralDiagramStarch.png|center|200px]] | |[[File:StructuralDiagramStarch.png|center|200px]] | ||
|- | |- | ||
| Line 68: | Line 68: | ||
|- | |- | ||
|[[File:StructuralDiagramGlycine.png|center|200px]] | |[[File:StructuralDiagramGlycine.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:StructuralDiagramPolyglycine.png|center|200px]] | |[[File:StructuralDiagramPolyglycine.png|center|200px]] | ||
|- | |- | ||
Revision as of 15:31, 19 January 2019
Key Stage 4
Meaning
Polymerisation is a chemical reaction in which small molecules known as monomers react to form a polymer.
About Polymerisation
Polymerisation may happen between:
- Identical monomers - Alkenes to Polyalkenes
- Two different monomers with complimantary functional groups at each end. - Esters to Polyesters
- Several different monomers of a homologous series - Peptides to Polypeptides.
Examples
| Ethene monomers can react together in an Addition Polymerisation reaction. | Polythene (sometimes spelled Polyethene) is formed. |
| Tetrafluoroethene monomers can react together in an Addition Polymerisation reaction. | Polytetrafluoroethene (sometimes referred to as PTFE or by a trademark TeflonTM) is formed. |
| Propene monomers can react together in an Addition Polymerisation reaction. | Polypropene is formed. |
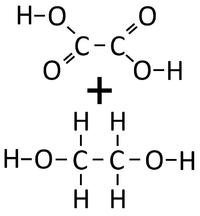
| Ethandioate and Ethandiol can react together in a Condensation Polymerisation. | A Polyester is formed along with Water. |
| Glucose molecules react together in a Condensation Polymerisation reaction. | Starch is formed along with Water. |
| Glycine molecules react together in a Condensation Polymerisation reaction. | A Polypeptide (Protein) is formed along with Water. In reality Polypeptides are made of many different Peptides (Amino Acids) rather than the same one repeated. |