Difference between revisions of "Chemical Formula"
(→About Chemical Formulae) |
|||
| Line 38: | Line 38: | ||
*CO<sub>2</sub>: [[Carbon Dioxide]] with one [[Carbon]] [[atom]] and two [[Oxygen]] [[atom]]s. | *CO<sub>2</sub>: [[Carbon Dioxide]] with one [[Carbon]] [[atom]] and two [[Oxygen]] [[atom]]s. | ||
*Li<sub>2</sub>O: [[Lithium Oxide]] with two [[Lithium]] [[atom]]s and one [[Oxygen]] [[atom]]. | *Li<sub>2</sub>O: [[Lithium Oxide]] with two [[Lithium]] [[atom]]s and one [[Oxygen]] [[atom]]. | ||
| + | H<sub>2</sub>SO<sub>4</sub>: [[Sulphuric Acid]] with two [[Hydrogen]] [[atom]]s, one [[Sulphur]] [[atom]] and four [[Oxygen]] [[atom]]s. | ||
| + | |||
| + | |||
| + | Some '''chemical formulae''' are written with the [[element]]s at specific points, not all grouped together, to show where different [[element]]s are [[Chemical Bond|bonded]]. | ||
| + | *CH<sub>3</sub>COOH: [[Ethanoic Acid]] with two [[Carbon]] [[atom]]s, two [[Oxygen]] [[atom]]s and four [[Hydrogen]] [[atom]]s. This shows that one [[Carbon]] is [[Chemical Bond|bonded]] to three [[Hydrogen]] [[atom]]s while the other [[Carbon]] is [[Double Bond|double bonded]] to one [[Oxygen]] and has a [[Single Bond|single bond]] with an [[Oxygen]], which itself it bonded to a [[Hydrogen]]. | ||
Revision as of 09:52, 7 December 2018
Contents
Key Stage 3
Meaning
A Chemical Formula is a simple way to show the number and type of atoms in a chemical.
About Chemical Formulae
- Chemical Formulae show which elements are in a molecule.
- The number of each element in a molecule is shown with a number written just after and lower than the element.
Examples
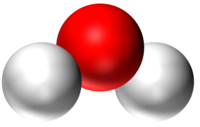
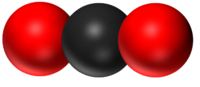
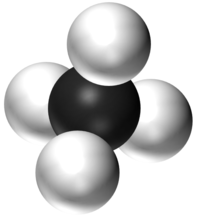
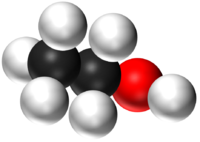
| H2O | CO2 | CH4 | CH5OH |
| Water has 2 Hydrogen atoms and 1 Oxygen atom. | Carbon Dioxide has 1 Carbon atom and 2 Oxygen atoms. | Methane has 1 Carbon atom and 4 Hydrogen atoms. | Ethanol has 2 Carbon atoms, 6 Hydrogen atoms and 1 Oxygen atom. |
Key Stage 4
Meaning
A Chemical Formula is a way to show the numbers of atoms of each element in a chemical.
About Chemical Formulae
- Chemical Formulae show which elements are in a molecule.
- The number of each element in a molecule is shown with subscript after that element.
- MgBr2: Magnesium Bromide with one Magnesium atom and two Bromine atoms.
- CO2: Carbon Dioxide with one Carbon atom and two Oxygen atoms.
- Li2O: Lithium Oxide with two Lithium atoms and one Oxygen atom.
H2SO4: Sulphuric Acid with two Hydrogen atoms, one Sulphur atom and four Oxygen atoms.
Some chemical formulae are written with the elements at specific points, not all grouped together, to show where different elements are bonded.