Difference between revisions of "Electron Orbital"
(→Meaning) |
|||
| Line 22: | Line 22: | ||
===Meaning=== | ===Meaning=== | ||
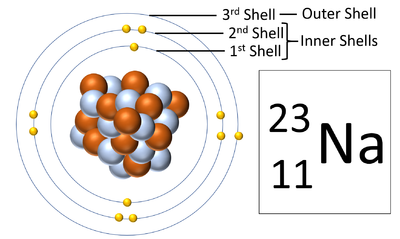
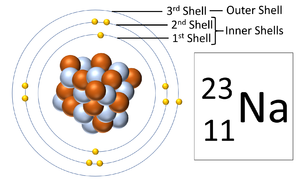
[[File:SodiumShells.png|right|300px|thumb|A [[diagram]] showing the three '''electron shells''' of a [[Sodium]] [[atom]].]] | [[File:SodiumShells.png|right|300px|thumb|A [[diagram]] showing the three '''electron shells''' of a [[Sodium]] [[atom]].]] | ||
| − | + | '''Electron orbitals''', also known as an '''electron shells''', are the locations where [[electron]]s [[orbit]] the [[Atomic Nucleus|nucleus]] of [[atom]]s. | |
===About Electron Orbitals=== | ===About Electron Orbitals=== | ||
Revision as of 12:32, 6 December 2018
Contents
Key Stage 3
Meaning
Electron shells are the places around a nucleus where an electron can orbit the nucleus.
| A diagram of a Sodium atom shown the electron shells. |
About Electron Shells
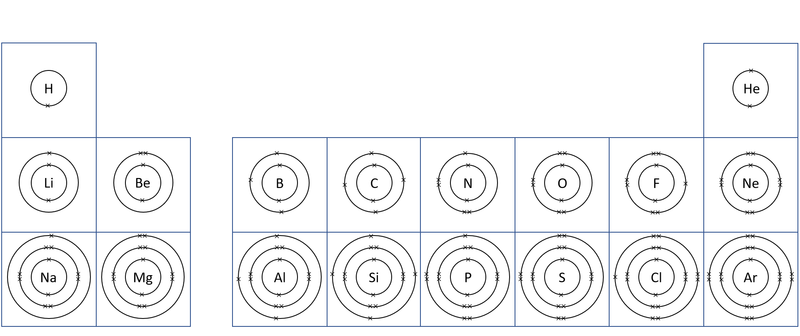
- The number of electron shells is shown by the period on the Periodic Table.
- The number of electrons in the outer shell determines the chemical properties of the element.
| A diagram of the first 20 elements in the Periodic Table showing the electron shells. |
Key Stage 4
Meaning
Electron orbitals, also known as an electron shells, are the locations where electrons orbit the nucleus of atoms.
About Electron Orbitals
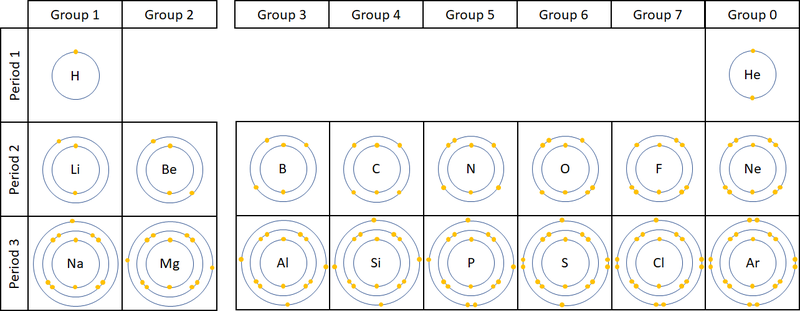
- Each electron orbital only holds a certain number of electrons.
- These orbitals and the number of electrons in an atom determine the chemistry of an element.
- The number of electron orbitals determines the Period on the Periodic Table.
- The number of electrons in the last orbital (Outer Shell) determines the Group on the Periodic Table.
| A diagram showing the electron shells and electrons in the first 20 elements on the Periodic Table. NB Group 0 used to be called Group 8 but this caused confusion because most elements in Group 8 have 8 electrons in their Outer Shell but Helium only has 2, so it was renamed Group 0. |
- Atoms in the same group have similar chemical properties because they all have the same number of electrons in their Outer Shell.