Difference between revisions of "Chemical Formula"
(→Examples) |
|||
| Line 12: | Line 12: | ||
===Examples=== | ===Examples=== | ||
{| class="wikitable" | {| class="wikitable" | ||
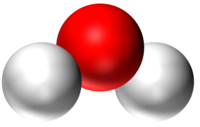
| − | | style="height:20px; width:200px; text-align:center;" |''' | + | | style="height:20px; width:200px; text-align:center;" |'''H<sub>2</sub>O''' |
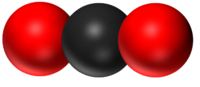
| − | | style="height:20px; width:200px; text-align:center;" |''' | + | | style="height:20px; width:200px; text-align:center;" |'''CO<sub>2</sub>''' |
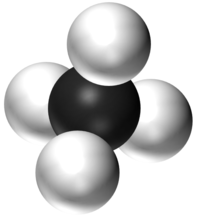
| − | | style="height:20px; width:200px; text-align:center;" |''' | + | | style="height:20px; width:200px; text-align:center;" |'''CH<sub>4</sub>''' |
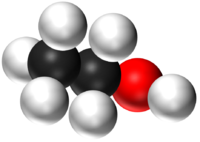
| − | | style="height:20px; width:200px; text-align:center;" |''' | + | | style="height:20px; width:200px; text-align:center;" |'''CH<sub>5</sub>OH''' |
|- | |- | ||
|[[File:WaterMolecule.png|center|200px]] | |[[File:WaterMolecule.png|center|200px]] | ||
| Line 22: | Line 22: | ||
|[[File:EthanolMolecule.png|center|200px]] | |[[File:EthanolMolecule.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |[[Water]] has 2 [[Hydrogen]] [[atom]]s and 1 [[Oxygen]] [[atom]]. |
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |[[Carbon Dioxide]] has 1 [[Carbon]] [[atom]] and 2 [[Oxygen]] [[atom]]s. |
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |[[Methane]] has 1 [[Carbon]] [[atom]] and 4 [[Hydrogen]] [[atom]]s. |
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |[[Ethanol]] has 2 [[Carbon]] [[atom]]s, 6 [[Hydrogen]] [[atom]]s and 1 [[Oxygen]] [[atom]]. |
|} | |} | ||
Revision as of 16:14, 23 September 2018
Key Stage 3
Meaning
A Chemical Formula is a simple way to show the number and type of atoms in a chemical.
About Chemical Formulae
- Chemical Formulae show which elements are in a molecule.
- The number of each element in a molecule is shown with a number written just after and lower than the element.
Examples
| H2O | CO2 | CH4 | CH5OH |
| Water has 2 Hydrogen atoms and 1 Oxygen atom. | Carbon Dioxide has 1 Carbon atom and 2 Oxygen atoms. | Methane has 1 Carbon atom and 4 Hydrogen atoms. | Ethanol has 2 Carbon atoms, 6 Hydrogen atoms and 1 Oxygen atom. |