Difference between revisions of "PH"
| Line 45: | Line 45: | ||
:[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d206 ''pH, pages 51-53, 108, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d206 ''pH, pages 51-53, 108, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
:[https://www.amazon.co.uk/gp/product/0008158754/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158754&linkCode=as2&tag=nrjc-21&linkId=27ad53b0283feeff7fc5ae04a9e205f418 ''pH, pages 97, 98-9, 101, 103, 348-9, GCSE Biology; Student Book, Collins, AQA ''] | :[https://www.amazon.co.uk/gp/product/0008158754/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158754&linkCode=as2&tag=nrjc-21&linkId=27ad53b0283feeff7fc5ae04a9e205f418 ''pH, pages 97, 98-9, 101, 103, 348-9, GCSE Biology; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''pH, pages 105, 106, 209, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''pH, pages 118, 120-124, 323, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''pH, pages 43, 44, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''pH; pH probes, page 110, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
Revision as of 02:24, 27 November 2019
Contents
Key Stage 3
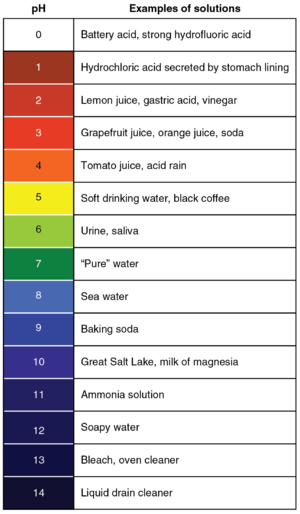
A pH scale with the colours of Universal Indicator at those pH values and some examples of substances at those pH values.
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About pH
- pH is written with a lower case p and an upper case H and refers to the 'power of Hydrogen' as there are free Hydrogen ions in an acid.
- Acids have a pH of less than 7 with the strongest acids being at pH 0.
- Bases have a pH greater than 7 with the strongest bases being at pH 14. Remember alkalis are a base dissolved in water.
- pH 7.0 is neutral.
Key Stage 4 Foundation
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About the pH Scale
- The pH of a solution is determined by the strength and concentration of Hydrogen ions in an acid or Hydroxide ions in an alkali.
- An acid will have a greater concentration of Hydrogen ions the lower on the pH scale.
- An alkali will have a greater concentration of Hydroxide ions the higher on the pH scale.
- A neutral solution will have an equal concentration of Hydrogen ions and Hydroxide ions and have a pH of 7.
Key Stage 4 Higher
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About the pH Scale
- The pH of a solution is determined by the strength and concentration of Hydrogen ions in an acid or Hydroxide ions in an alkali.
- An acid will have a greater concentration of Hydrogen ions the lower on the pH scale.
- An alkali will have a greater concentration of Hydroxide ions the higher on the pH scale.
- A neutral solution will have an equal concentration of Hydrogen ions and Hydroxide ions and have a pH of 7.
- In acids for a pH decrease of 1 there must be 10 times greater concentration of Hydrogen ions in solution.
References
AQA
- pH scale, pages 107-8, GCSE Chemistry, Hodder, AQA
- Ph scale, pages 209-10, GCSE Combined Science Trilogy 1, Hodder, AQA
- pH scale, pages 96-99, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- pH, pages 124, 125, 128, 129, 237, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- pH, pages 128, 234, GCSE Combined Science; The Revision Guide, CGP, AQA
- pH, pages 131, 148-9, 153, 167, 328-9, GCSE Chemistry; Student Book, Collins, AQA
- pH, pages 146, 147, 150, 153, 154, 315, GCSE Chemistry, CGP, AQA
- pH, pages 45-48, GCSE Biology; Third Edition, Oxford University Press, AQA
- pH, pages 51-53, 108, GCSE Chemistry; The Revision Guide, CGP, AQA
- pH, pages 97, 98-9, 101, 103, 348-9, GCSE Biology; Student Book, Collins, AQA