Difference between revisions of "Energy Level"
| Line 36: | Line 36: | ||
:[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Energy level diagrams, pages 155, 156, 167, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Energy level diagrams, pages 155, 156, 167, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
:[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Energy level diagrams, pages 180, 181, 199, GCSE Chemistry, CGP, AQA ''] | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Energy level diagrams, pages 180, 181, 199, GCSE Chemistry, CGP, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Energy levels in atoms, pages 128, 151, 153, 154, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Energy levels, pages 49, 50, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | ||
Revision as of 13:49, 19 November 2019
Key Stage 4
Meaning
Energy Levels are another name for the electron shells or orbitals around the nucleus where electrons can exist.
About Energy Levels
- The existence of energy levels in atoms is part of the Bohr model of the atom.
- The electron orbitals in atoms each correspond to electrons with a certain amount of energy, which is why they are also called energy levels.
- Electrons cannot exist anywhere between the energy levels they can only exist in one energy level or another.
- In chemistry electrons are seen as fixed in their energy levels but in physics the electrons can move to a higher energy level by the absorption of energy and can drop down into an empty energy level below by emitting energy.
- The wavelengths of electromagnetic wave depend on the energy difference between the energy levels in atoms.
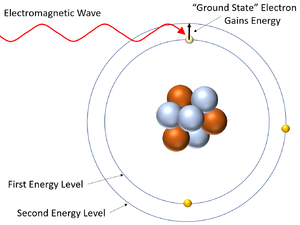
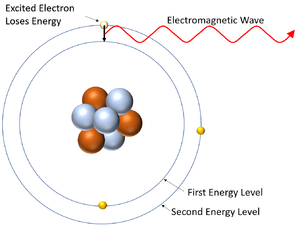
| This diagram shows an electron gaining energy by absorbing an electromagnetic wave and moving to a higher energy level (becoming excited). | This diagram shows an excited electron losing energy by emitting an electromagnetic wave. As it does this the electron falls back down to a lower energy level. |
- If an electron in an the highest energy level, known as the outer shell, gains enough energy it can leave the atom completely so they atom becomes a positive ion.
References
AQA
- Energy level of electrons, pages 13, 18-19, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Energy levels (atoms), page 197, GCSE Combined Science; The Revision Guide, CGP, AQA
- Energy levels (electron shells), pages 22, 43-45, GCSE Chemistry, CGP, AQA
- Energy levels (electron shells), pages 22, 43-45, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Energy levels (shells), page 117-18, GCSE Combined Science Trilogy 1, Hodder, AQA
- Energy levels (shells), pages 2,3,5, GCSE Chemistry, Hodder, AQA
- Energy levels in atoms, page 43, GCSE Physics; The Revision Guide, CGP, AQA
- Energy levels in atoms, pages 111, 201, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Energy levels in atoms, pages 123, 243, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Energy level diagrams, pages 155, 156, 167, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Energy level diagrams, pages 180, 181, 199, GCSE Chemistry, CGP, AQA