Difference between revisions of "Emission Spectra"
| Line 23: | Line 23: | ||
:[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Emission spectra, pages 214-15, GCSE Chemistry, Hodder, AQA ''] | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Emission spectra, pages 214-15, GCSE Chemistry, Hodder, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Emission spectra, page 195, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Emission spectrum, page 358, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Emission spectrum, page 94, GCSE Physics, Pearson Edexcel ''] | ||
Latest revision as of 11:53, 19 November 2019
Contents
Key Stage 4
Meaning
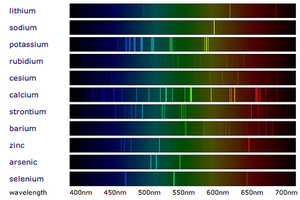
Emission spectra are the specific wavelengths of light emitted by the electrons in atoms as they lose energy.
About Emission Spectra
- An emission spectrum is made by providing energy to a material and focusing any light emitted through a prism to separate the colours.
- The spectrum of white light is a continuous change of colours with all wavelengths having the same intensity.
- An emission spectrum is a set of specific wavelengths with a high intensity. This appears as bright lines of colour on a spectrum.
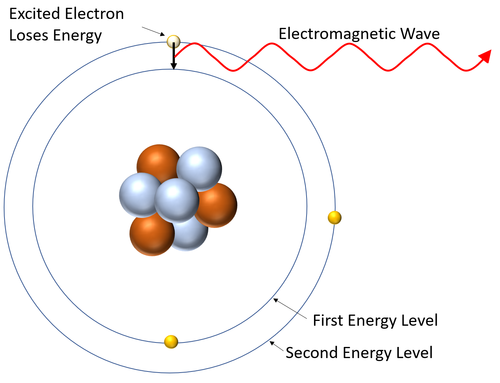
- A emission spectrum is created when excited electrons (electrons in high energy levels) lose energy and fall to a lower energy level emitting a specific wavelength of electromagnetic wave when they do.
- The wavelengths of electromagnetic wave depend on the energy difference between the energy levels in atoms.
| This diagram shows an excited electron losing energy by emitting an electromagnetic wave. As it does this the electron falls back down to a lower energy level. |