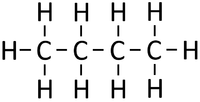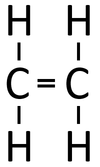Difference between revisions of "Cracking"
(→Examples) |
|||
| Line 36: | Line 36: | ||
Decane → Hexane + Butene | Decane → Hexane + Butene | ||
: <chem>C10H34 -> C6H14 + C4H8</chem> | : <chem>C10H34 -> C6H14 + C4H8</chem> | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09814 ''Cracking (of hydrocarbons), page 149, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Cracking (of hydrocarbons), page 226, 227, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d96 ''Cracking (of hydrocarbons), page 77, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Cracking (of hydrocarbons), pages 194, 195, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
Revision as of 23:08, 3 November 2019
Key Stage 4
Meaning
Cracking is a Thermal Decomposition process in which large hydrocarbon molecules are broken into smaller hydrocarbon molecules.
About Cracking
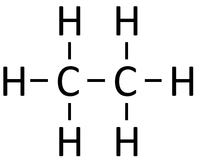
- Cracking is often done because Crude Oil contains more large hydrocarbon molecules than can be used and not enough short hydrocarbon molecules than are needed.
- When Crude Oil fractions are cracked the long alkanes are broken down into smaller alkanes and alkenes.
- Cracking is done at very high temperatures (500°C) and uses either a catalyst to aid the reaction or steam.
- When an alkane is cracked into smaller pieces there are not enough Hydrogen [[atom]s to produce two saturated hydrocarbons. One of the hydrocarbons must be unsaturated and therefor will have a double bond.
Examples
| If butane were cracked. | An alkane and an alkene are produced. | |||
Cracking a hydrocarbon can produce many possible products:
Hexadecane → Decane + Hexene
- <chem>C16H34 -> C10H22 + C6H12</chem>
Hexadecane → Decane + Butene + Ethene
- <chem>C16H34 -> C10H22 + C4H8 + C2H4</chem>
Decane → Pentane + Pentene
- <chem>C10H34 -> C5H12 + C5H10</chem>
Decane → Hexane + Butene
- <chem>C10H34 -> C6H14 + C4H8</chem>
References
AQA
- Cracking (of hydrocarbons), page 149, GCSE Combined Science; The Revision Guide, CGP, AQA
- Cracking (of hydrocarbons), page 226, 227, GCSE Chemistry, CGP, AQA
- Cracking (of hydrocarbons), page 77, GCSE Chemistry; The Revision Guide, CGP, AQA
- Cracking (of hydrocarbons), pages 194, 195, GCSE Combined Science Trilogy; Chemistry, CGP, AQA