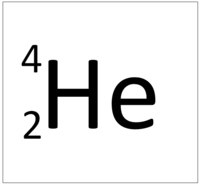Difference between revisions of "Atomic Number"
| Line 83: | Line 83: | ||
:[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Atomic (proton) number, pages 79, 174, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Atomic (proton) number, pages 79, 174, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
:[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Atomic (proton), number, page 51, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Atomic (proton), number, page 51, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Atomic number, page 20, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Atomic number, page 92, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Atomic number, pages 164, 356, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Atomic number; periodic table, pages 172-173, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Atomic number; periodic table, pages 28-29, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Atomic numbers, page 16, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Atomic numbers, pages 35, 36, GCSE Chemistry, CGP, Edexcel ''] | ||
Revision as of 11:37, 29 October 2019
Contents
Key Stage 3
Meaning
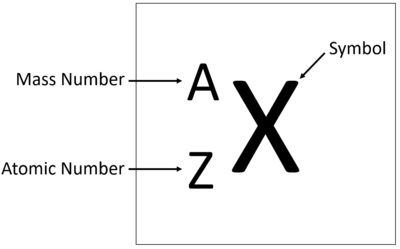
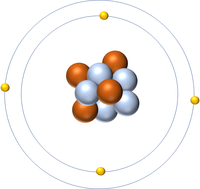
The Atomic Number is the number of protons in the nucleus of an atom.
About The Atomic Number
- The Atomic Number of an atom determines which element it is.
- The number of protons also determines the number of electrons.
Examples
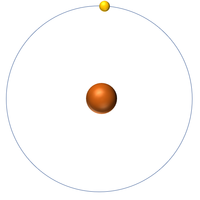
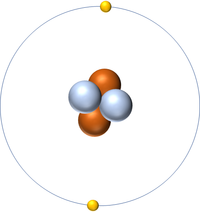
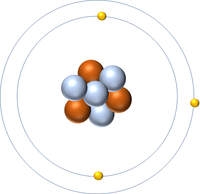
| Hydrogen | Helium | Lithium | Beryllium |
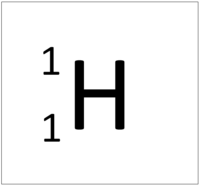
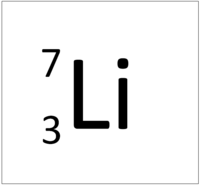
| Hydrogen has 1 proton so its atomic number is 1. | Helium has 2 protons so its atomic number is 2. | Lithium has 3 protons so its atomic number is 3. | Beryllium has 4 protons so its atomic number is 4. |
Key Stage 4
Meaning
The Atomic Number is the number of protons in the nucleus of an atom.
About The Atomic Number
- The Atomic Number of an atom determines which element it is.
- Protons have a relative atomic charge of +1 so the number of protons determines the relative atomic charge of the atomic nucleus.
- The number of electrons orbiting the nucleus is the same as the number of protons in the nucleus of an atom.
Examples
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has 1 proton so its atomic number is 1 and the relative atomic charge of the nucleus is +1. | Helium has 2 protons so its atomic number is 2 and the relative atomic charge of the nucleus is +2. | Lithium has 3 protons so its atomic number is 3 and the relative atomic charge of the nucleus is +3. | Beryllium has 4 protons so its atomic number is 4 and the relative atomic charge of the nucleus is +4. |
References
AQA
- Atomic number (Z), page 89, GCSE Physics, Hodder, AQA
- Atomic number, page 111, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Atomic number, page 123, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Atomic number, page 3-4, GCSE Chemistry, Hodder, AQA
- Atomic number, page 44, GCSE Physics; The Revision Guide, CGP, AQA
- Atomic number, pages 119, 339, GCSE Combined Science Trilogy 1, Hodder, AQA
- Atomic numbers, page 96, GCSE Physics; Third Edition, Oxford University Press, AQA
- Atomic numbers, pages 12, 13, 22, GCSE Chemistry; The Revision Guide, CGP, AQA
- Atomic numbers, pages 14, 16, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atomic numbers, pages 25, 52, GCSE Chemistry, CGP, AQA
- Atomic numbers, pages 25, 52, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Atomic numbers, pages 96, 97, 107, 198, 199, GCSE Combined Science; The Revision Guide, CGP, AQA
- Atomic; number, pages 109, 111, 116-17, GCSE Physics; Student Book, Collins, AQA
Edexcel
- Atomic (proton) number, pages 79, 174, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Atomic (proton), number, page 51, GCSE Physics; The Revision Guide, CGP, Edexcel
- Atomic number, page 20, GCSE Chemistry, Pearson, Edexcel
- Atomic number, page 92, GCSE Physics, Pearson Edexcel
- Atomic number, pages 164, 356, GCSE Combined Science, Pearson Edexcel
- Atomic number; periodic table, pages 172-173, GCSE Combined Science, Pearson Edexcel
- Atomic number; periodic table, pages 28-29, GCSE Chemistry, Pearson, Edexcel
- Atomic numbers, page 16, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Atomic numbers, pages 35, 36, GCSE Chemistry, CGP, Edexcel