Difference between revisions of "Flame Emission Spectroscopy"
(→About Flame Emission Spectroscopy) |
|||
| Line 1: | Line 1: | ||
==Key Stage 4== | ==Key Stage 4== | ||
===Meaning=== | ===Meaning=== | ||
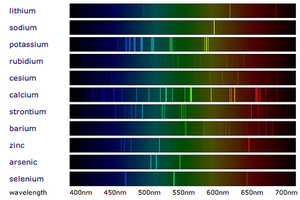
| − | [[File:FlameEmissionSpectroscopy.png|right|300px|thumb|The [[ | + | [[File:FlameEmissionSpectroscopy.png|right|300px|thumb|The [[Emission Spectra|line spectra]] from the '''flame emission spectroscopy''' of several [[metal]]s.]] |
'''Flame emission spectroscopy''' is a technique for identifying [[metal]]s in a [[metal]] [[compound]]. | '''Flame emission spectroscopy''' is a technique for identifying [[metal]]s in a [[metal]] [[compound]]. | ||
Revision as of 12:32, 8 April 2019
Key Stage 4
Meaning
Flame emission spectroscopy is a technique for identifying metals in a metal compound.
About Flame Emission Spectroscopy
- Flame emission spectroscopy is an advanced version of the Flame Tests and uses a Spectroscope to separate the colours into a spectrum.
- When white light passes through a Spectroscope the colours are split into a spectrum (the rainbow). When metal compounds burn they only produce certain colours so when this light is passed through a spectroscope in flame emission spectroscopy it produces very specific lines called a 'Line Spectrum' instead of the broad spectrum seen from white light.
- Metals in a metal compound can be identified by comparing it to the line spectra of known metals.