Difference between revisions of "Flame Emission Spectroscopy"
| Line 6: | Line 6: | ||
===About Flame Emission Spectroscopy=== | ===About Flame Emission Spectroscopy=== | ||
: '''Flame emission spectroscopy''' is an advanced version of the [[Flame Test]]s and uses a [[Spectroscope]] to separate the colours into a [[spectrum]]. | : '''Flame emission spectroscopy''' is an advanced version of the [[Flame Test]]s and uses a [[Spectroscope]] to separate the colours into a [[spectrum]]. | ||
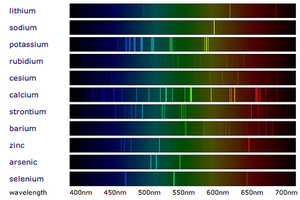
| − | : When [[White Light|white light]] passes through a [[Spectroscope]] the colours are split into a [[spectrum]] (the rainbow). When [[Metal Compound|metal compounds]] burn they only produce certain colours so when this [[light]] is passed through a [[spectroscope]] in '''flame emission spectroscopy''' it produces very specific lines called a '[[Line Spectrum]]' instead of the broad [[spectrum]] seen from [[White Light|white light]]. | + | : When [[White Light|white light]] passes through a [[Spectroscope]] the colours are split into a [[spectrum]] (the rainbow). When [[Metal Compound|metal compounds]] burn they only produce certain colours so when this [[light]] is passed through a [[spectroscope]] in '''flame emission spectroscopy''' it produces very specific lines called a '[[Emission Spectra|Line Spectrum]]' instead of the broad [[spectrum]] seen from [[White Light|white light]]. |
: [[Metal]]s in a [[metal]] [[compound]] can be identified by comparing it to the [[Line Spectrum|line spectra]] of known [[metal]]s. | : [[Metal]]s in a [[metal]] [[compound]] can be identified by comparing it to the [[Line Spectrum|line spectra]] of known [[metal]]s. | ||
Revision as of 18:03, 7 April 2019
Key Stage 4
Meaning
Flame emission spectroscopy is a technique for identifying metals in a metal compound.
About Flame Emission Spectroscopy
- Flame emission spectroscopy is an advanced version of the Flame Tests and uses a Spectroscope to separate the colours into a spectrum.
- When white light passes through a Spectroscope the colours are split into a spectrum (the rainbow). When metal compounds burn they only produce certain colours so when this light is passed through a spectroscope in flame emission spectroscopy it produces very specific lines called a 'Line Spectrum' instead of the broad spectrum seen from white light.
- Metals in a metal compound can be identified by comparing it to the line spectra of known metals.