Difference between revisions of "Red Cabbage Indicator"
| Line 20: | Line 20: | ||
===Making Red Cabbage Indicator=== | ===Making Red Cabbage Indicator=== | ||
| − | : 1. Tear 20g of red cabbage leaf into small pieces and place into a | + | : 1. Tear 20g of red cabbage leaf into small pieces and place into a 500ml [[beaker]]. |
| − | : 2. Add | + | : 2. Add 300ml of hot [[water]] to the [[beaker]]. |
: 3. Stir with a [[Glass Rod|glass rod]] for 3 minutes. | : 3. Stir with a [[Glass Rod|glass rod]] for 3 minutes. | ||
: 4. Place a [[funnel]] in a [[Conical Flask|conical flask]] and place some [[Filter Paper|filter paper]] in the [[funnel]]. | : 4. Place a [[funnel]] in a [[Conical Flask|conical flask]] and place some [[Filter Paper|filter paper]] in the [[funnel]]. | ||
: 5. [[Filtration|Filter]] the red cabbage and water [[mixture]] by pouring through the [[Filter Paper|filter paper]] into the [[Conical Flask|conical flask]]. | : 5. [[Filtration|Filter]] the red cabbage and water [[mixture]] by pouring through the [[Filter Paper|filter paper]] into the [[Conical Flask|conical flask]]. | ||
: 6. The [[filtrate]] is your '''red cabbage indicator'''. You can use a [[pipette]] to add small amounts to [[solution]]s to find their [[pH]]. | : 6. The [[filtrate]] is your '''red cabbage indicator'''. You can use a [[pipette]] to add small amounts to [[solution]]s to find their [[pH]]. | ||
Latest revision as of 15:26, 6 April 2019
Key Stage 3
Meaning
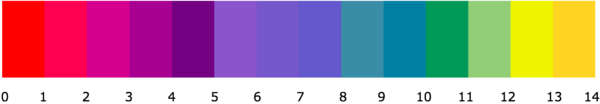
Red cabbage indicator is a pH indicator made from the leaves of a red cabbage.
About Red Cabbage Indicator
Making Red Cabbage Indicator
- 1. Tear 20g of red cabbage leaf into small pieces and place into a 500ml beaker.
- 2. Add 300ml of hot water to the beaker.
- 3. Stir with a glass rod for 3 minutes.
- 4. Place a funnel in a conical flask and place some filter paper in the funnel.
- 5. Filter the red cabbage and water mixture by pouring through the filter paper into the conical flask.
- 6. The filtrate is your red cabbage indicator. You can use a pipette to add small amounts to solutions to find their pH.