Difference between revisions of "Group 5"
(→About Group 5) |
(→Key Stage 4) |
||
| Line 31: | Line 31: | ||
:*[[Nitrogen]] can gain 3 [[electron]]s to form [[Negative Ion|negative ions]] with a [[Electrical Charge|charge]] of -3. | :*[[Nitrogen]] can gain 3 [[electron]]s to form [[Negative Ion|negative ions]] with a [[Electrical Charge|charge]] of -3. | ||
:*[[Phosphorus]] can gain 3 [[electron]]s to form [[Negative Ion|negative ions]] with a [[Electrical Charge|charge]] of -3. | :*[[Phosphorus]] can gain 3 [[electron]]s to form [[Negative Ion|negative ions]] with a [[Electrical Charge|charge]] of -3. | ||
| − | :*[[Arsenic]] can | + | :*[[Arsenic]] can gain 3 [[electron]]s to form [[Negative Ion|negative ions]] with a [[Electrical Charge|charge]] of -3. |
:*[[Antimony]] can lose [[electron]]s to form [[Positive Ion|positive ions]]. | :*[[Antimony]] can lose [[electron]]s to form [[Positive Ion|positive ions]]. | ||
:*[[Bismuth]] can lose [[electron]]s to form [[Positive Ion|positive ions]]. | :*[[Bismuth]] can lose [[electron]]s to form [[Positive Ion|positive ions]]. | ||
Latest revision as of 08:43, 4 April 2019
Key Stage 3
Meaning
Group 5 are elements on the Periodic Table with 5 electrons in their outer shell.
About Group 5
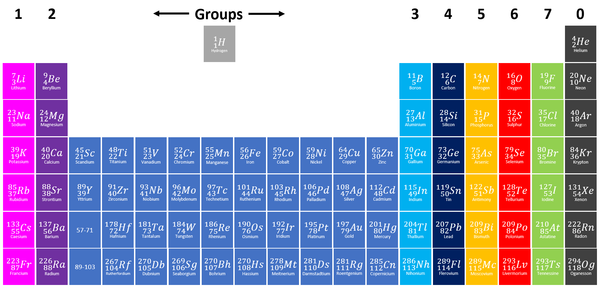
| Group 5 elements are shown in yellow on this Periodic Table. |
Key Stage 4
Meaning
Group 5 are elements on the Periodic Table with 5 electrons in their outer shell.
About Group 5
| Group 5 elements are shown in yellow on this Periodic Table. |
- The elements in Group 5 are:
- Nitrogen can gain 3 electrons to form negative ions with a charge of -3.
- Phosphorus can gain 3 electrons to form negative ions with a charge of -3.
- Arsenic can gain 3 electrons to form negative ions with a charge of -3.
- Antimony can lose electrons to form positive ions.
- Bismuth can lose electrons to form positive ions.