Difference between revisions of "Isotope"
(→Calculating the Average Relative Atomic Mass) |
(→Isotopic Abundance) |
||
| Line 36: | Line 36: | ||
: The [[Periodic Table]] orders the [[element]]s due to their [[Chemical Property|chemical properties]] but since [[isotope]]s of the same [[element]] have the same [[Chemical Property|chemical properties]] then different [[isotope]]s are not included. | : The [[Periodic Table]] orders the [[element]]s due to their [[Chemical Property|chemical properties]] but since [[isotope]]s of the same [[element]] have the same [[Chemical Property|chemical properties]] then different [[isotope]]s are not included. | ||
: On the [[Periodic Table]] an [[average]] [[Relative Atomic Mass|atomic mass]] is given based on the [[Relative Atomic Mass|mass]] of each [[isotope]] and how common it is. | : On the [[Periodic Table]] an [[average]] [[Relative Atomic Mass|atomic mass]] is given based on the [[Relative Atomic Mass|mass]] of each [[isotope]] and how common it is. | ||
| + | : To find the [[Mean Average|average]] [[Relative Atomic Mass|atomic mass]] the [[Relative Atomic Mass|mass]] of each [[isotope]] is considered along with the [[ratio]] of abundance. | ||
====Calculating the Average Relative Atomic Mass==== | ====Calculating the Average Relative Atomic Mass==== | ||
| − | |||
| − | + | {| class="wikitable" | |
| + | | style="height:20px; width:300px; text-align:center;" |[[Chlorine]] has two common [[Stable Isotope|stable]] [[Isotope]]s; [[Chlorine-35]] and [[Chlorine-37]]. [[Chlorine-35]] is three times more common than [[Chlorine-37]] so the ratio is 3:1. Find the [[Mean Average|average]] [[Relative Atomic Mass|atomic mass]] of [[Chlorine]]. | ||
| + | | style="height:20px; width:300px; text-align:center;" |[[Lithium]] has two common [[Stable Isotope|stable]] [[Isotope]]s; [[Lithium-7]] and [[Lithium-6]]. | ||
| + | 92.5% of all [[Lithium]] is the [[isotope]] [[Lithium-7]] while [[Lithium-6]] makes up only 7.5%. Find the [[Mean Average|average]] [[Relative Atomic Mass|atomic mass]] of [[Lithium]]. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s.''' | ||
Ratio: 3:1 | Ratio: 3:1 | ||
3xChlorine-35 : 1xChlorine-37 | 3xChlorine-35 : 1xChlorine-37 | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s.''' | ||
| + | |||
| + | Ratio: 92.5:7.5 | ||
| + | |||
| + | 92.5xLithium-7 : 7.5xLithium-6 | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers into the [[equation]] and [[Solve (Maths)|solve]].''' | ||
To find the [[Mean Average|average]]: | To find the [[Mean Average|average]]: | ||
| − | |||
| − | |||
| − | |||
| − | + | <math>Average=\frac{35+35+35+37}{4}</math> | |
| − | + | <math>Average=\frac{35 \times 3 + 37 \times 1}{4}</math> | |
| − | |||
| − | + | <math>Average=35.5</math> | |
| − | + | So the [[Relative Atomic Mass]] of [[Chlorine]] is quoted as 35.5 on the [[Periodic Table]]. | |
| + | | style="height:20px; width:300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers into the [[equation]] and [[Solve (Maths)|solve]].''' | ||
To find the [[Mean Average|average]]: | To find the [[Mean Average|average]]: | ||
| − | + | ||
| − | + | <math>Average=\frac{7 \times 92.5 + 6 \times 7.5}{100}</math> | |
| + | |||
| + | <math>Average=6.925</math> | ||
So the [[Relative Atomic Mass]] of [[Lithium]] is quoted as 6.9, 6.93 or 6.925 depending on the [[precision]] of that particular [[Periodic Table]]. | So the [[Relative Atomic Mass]] of [[Lithium]] is quoted as 6.9, 6.93 or 6.925 depending on the [[precision]] of that particular [[Periodic Table]]. | ||
| + | |} | ||
Revision as of 09:07, 7 March 2019
Contents
Key Stage 4
Meaning
Isotopes are atoms with the same number of protons (the same element) but a different number of neutrons.
About Isotopes
- Each element has many isotopes but some are stable and others are unstable so they decay quickly into other isotopes or a different element.
- Different isotopes of the same element have the same atomic number but different atomic mass due to the different numbers of neutrons.
- Different isotopes of the same element have the same chemical properties but they have different physical properties. This means they cannot be separated by chemical processes but they can be separated by physical ones.
- Different isotopes of the same element may have different boiling points, different melting points, may diffuse at different rates and, as has already been stated, have a different mass. This means isotopes can be separated by processes such as Distillation, Chromatography and Centrifuge.
Examples
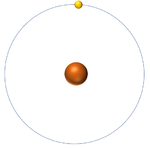
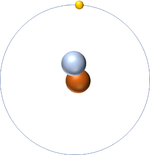
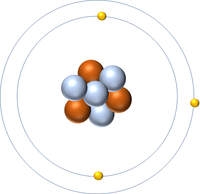
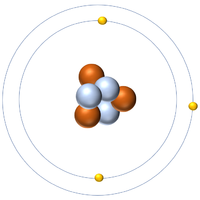
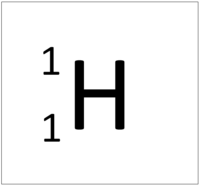
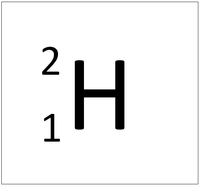
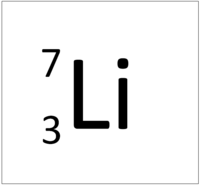
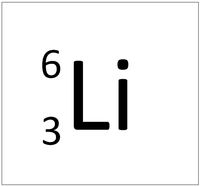
| Hydrogen-1 | Hydrogen-2 | Lithium-7 | Lithium-6 |
| Hydrogen always has 1 proton but isotope there are no neutrons. | Hydrogen always has 1 proton but in this isotope there is 1 neutron. This isotope of Hydrogen is known as Deuterium. | Lithium always has 3 protons but in this isotope there are 4 neutrons. | Lithium always has 3 protons but in this isotope there are 3 neutrons. |
Isotopic Abundance
- Different isotopes of the same element do not all appear in the same quantities. Some isotopes are more common than others.
- The Periodic Table orders the elements due to their chemical properties but since isotopes of the same element have the same chemical properties then different isotopes are not included.
- On the Periodic Table an average atomic mass is given based on the mass of each isotope and how common it is.
- To find the average atomic mass the mass of each isotope is considered along with the ratio of abundance.
Calculating the Average Relative Atomic Mass
| Chlorine has two common stable Isotopes; Chlorine-35 and Chlorine-37. Chlorine-35 is three times more common than Chlorine-37 so the ratio is 3:1. Find the average atomic mass of Chlorine. | Lithium has two common stable Isotopes; Lithium-7 and Lithium-6.
92.5% of all Lithium is the isotope Lithium-7 while Lithium-6 makes up only 7.5%. Find the average atomic mass of Lithium. |
| 1. State the known quantities in SI Units.
Ratio: 3:1 3xChlorine-35 : 1xChlorine-37 |
1. State the known quantities in SI Units.
Ratio: 92.5:7.5 92.5xLithium-7 : 7.5xLithium-6 |
| 2. Substitute the numbers into the equation and solve.
To find the average\[Average=\frac{35+35+35+37}{4}\] \(Average=\frac{35 \times 3 + 37 \times 1}{4}\) \(Average=35.5\) So the Relative Atomic Mass of Chlorine is quoted as 35.5 on the Periodic Table. |
2. Substitute the numbers into the equation and solve.
To find the average\[Average=\frac{7 \times 92.5 + 6 \times 7.5}{100}\] \(Average=6.925\) So the Relative Atomic Mass of Lithium is quoted as 6.9, 6.93 or 6.925 depending on the precision of that particular Periodic Table. |