Difference between revisions of "Electrostatic Force"
(→Key Stage 3) |
|||
| Line 27: | Line 27: | ||
|} | |} | ||
| − | ==Key Stage | + | ==Key Stage 4== |
===Meaning=== | ===Meaning=== | ||
The '''electrostatic force''' is a [[force]] that causes [[Electrical Charge|charged]] [[object]]s to be [[attract|attracted]] or [[repel|repelled]] by one another. | The '''electrostatic force''' is a [[force]] that causes [[Electrical Charge|charged]] [[object]]s to be [[attract|attracted]] or [[repel|repelled]] by one another. | ||
Revision as of 12:29, 11 February 2019
Contents
Key Stage 3
Meaning
The electrostatic force is a force that causes charged objects to be attracted or repelled by one another.
About The Electrostatic Force
- Electrostatic Force is a force so it is measured in Newtons.
- Electrostatic Force is a non-contact force because it can act without objects touching.
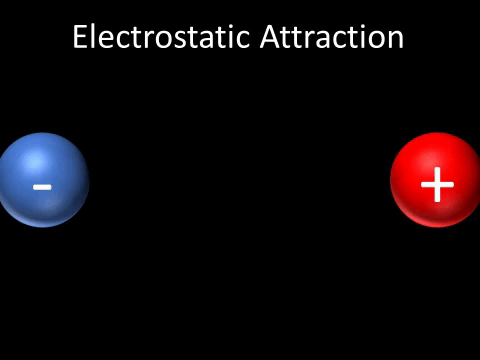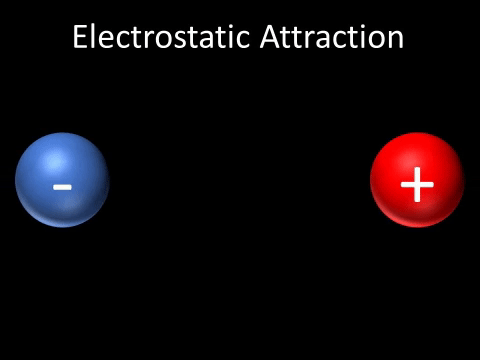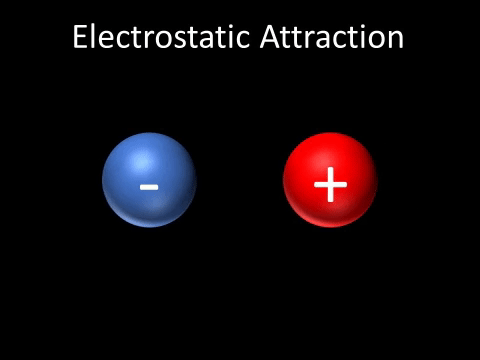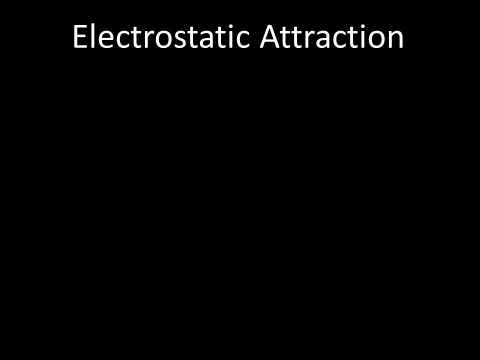
| A positively charged and negatively charged object will be attracted to each other. |
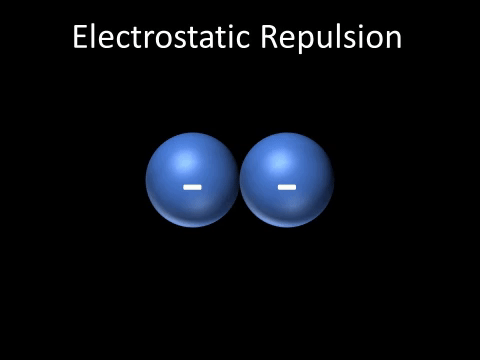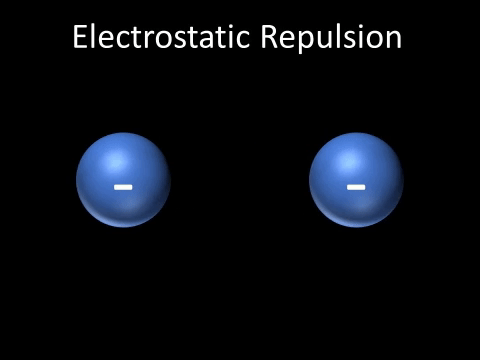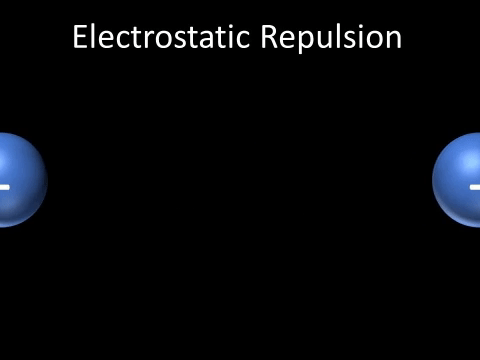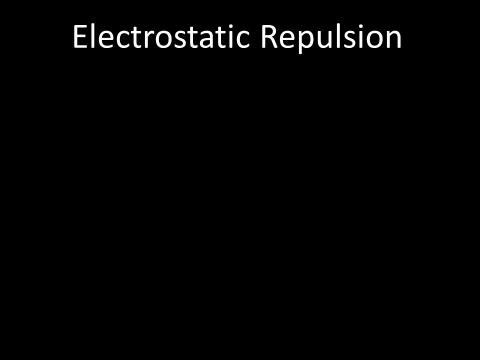
| Two negatively charged objects repel each other. |
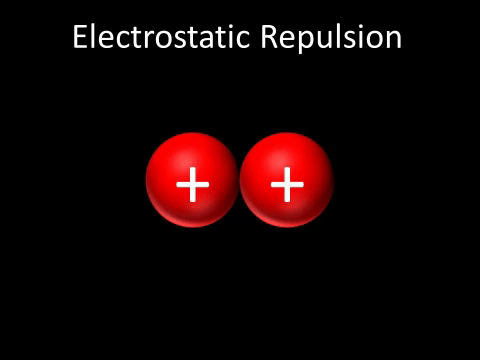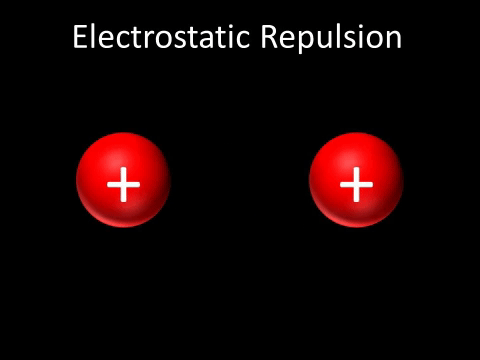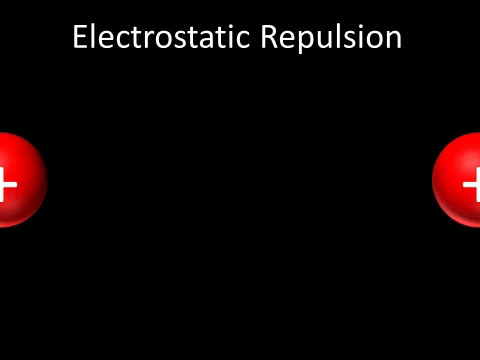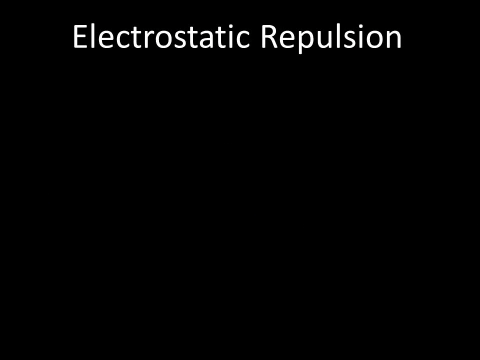
| Two positively charged objects repel each other. |
Key Stage 4
Meaning
The electrostatic force is a force that causes charged objects to be attracted or repelled by one another.
About The Electrostatic Force
- Electrostatic Force is a force so it is measured in Newtons.
- Electrostatic Force is a non-contact force because it can act without objects touching.
- A positively charged and negatively charged object will be attracted to each other.
- Two negatively charged objects repel each other.
- Two positively charged objects repel each other.
- The electrostatic force is what holds ions together in an ionic bond.
- Protons in the nucleus experience the electrostatic force of repulsion and in order for fusion to occur protons must have enough energy to overcome this force of repulsion.