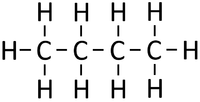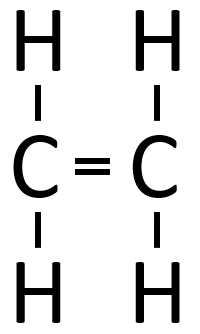Difference between revisions of "Cracking"
(Created page with "==Key Stage 4== ===Meaning=== Cracking is a Thermal Decomposition process in which large hydrocarbon molecules are broken into smaller hydrocarbon molecu...") |
(→Examples) |
||
| Line 13: | Line 13: | ||
|- | |- | ||
|[[File:StructuralDiagramButane.png|center|200px]] | |[[File:StructuralDiagramButane.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
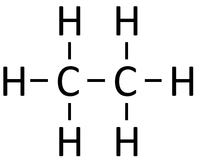
|[[File:StructuralDiagramEthane.png|center|200px]] | |[[File:StructuralDiagramEthane.png|center|200px]] | ||
| − | |[[File:AdditionSign.png|center| | + | |[[File:AdditionSign.png|center|100px]] |
|[[File:StructuralDiagramEthene.png|center|200px]] | |[[File:StructuralDiagramEthene.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |If [[butane]] were '''cracked'''. |
| | | | ||
| − | |colspan="3 | + | |colspan="3"|An [[alkane]] and an [[alkene]] are [[Product|produced]]. |
|} | |} | ||
Revision as of 10:18, 25 January 2019
Key Stage 4
Meaning
Cracking is a Thermal Decomposition process in which large hydrocarbon molecules are broken into smaller hydrocarbon molecules.
About Cracking
- Cracking is often done because Crude Oil contains more large hydrocarbon molecules than can be used and not enough short hydrocarbon molecules than are needed.
- When Crude Oil fractions are cracked the long alkanes are broken down into smaller alkanes and alkenes.
- Cracking is done at very high temperatures (500°C) and uses either a catalyst to aid the reaction or steam.
- When an alkane is cracked into smaller pieces there are not enough Hydrogen [[atom]s to produce two saturated hydrocarbons. One of the hydrocarbons must be unsaturated and therefor will have a double bond.
Examples
| If butane were cracked. | An alkane and an alkene are produced. | |||