Difference between revisions of "Polymerisation"
(→Examples) |
|||
| Line 16: | Line 16: | ||
|[[File:PolytheneFormula.png|center|200px]] | |[[File:PolytheneFormula.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Ethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an | + | | style="height:20px; width:200px; text-align:center;" |[[Ethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an [[Addition Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Polythene]] (sometimes spelled Polyethene) is formed. | | style="height:20px; width:200px; text-align:center;" |[[Polythene]] (sometimes spelled Polyethene) is formed. | ||
| Line 27: | Line 27: | ||
|[[File:PolyTetraFluoroEtheneFormula.png|center|200px]] | |[[File:PolyTetraFluoroEtheneFormula.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Tetrafluoroethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an | + | | style="height:20px; width:200px; text-align:center;" |[[Tetrafluoroethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an [[Addition Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Polytetrafluoroethene]] (sometimes referred to as PTFE or by a [[trademark]] Teflon<sup>TM</sup>) is formed. | | style="height:20px; width:200px; text-align:center;" |[[Polytetrafluoroethene]] (sometimes referred to as PTFE or by a [[trademark]] Teflon<sup>TM</sup>) is formed. | ||
| Line 38: | Line 38: | ||
|[[File:PolyPropeneFormula.png|center|200px]] | |[[File:PolyPropeneFormula.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Propene]] [[monomer]]s can [[Chemical Reaction|react]] together in an | + | | style="height:20px; width:200px; text-align:center;" |[[Propene]] [[monomer]]s can [[Chemical Reaction|react]] together in an [[Addition Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Polypropene]] is formed. | | style="height:20px; width:200px; text-align:center;" |[[Polypropene]] is formed. | ||
| Line 49: | Line 49: | ||
|[[File:StructuralDiagramPolyester.png|center|200px]] | |[[File:StructuralDiagramPolyester.png|center|200px]] | ||
|- | |- | ||
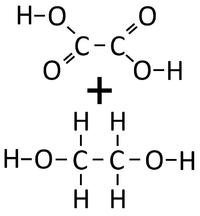
| − | | style="height:20px; width:200px; text-align:center;" |Ethandioate and Ethandiol can [[Chemical Reaction|react]] together in a | + | | style="height:20px; width:200px; text-align:center;" |Ethandioate and Ethandiol can [[Chemical Reaction|react]] together in a [[Condensation Polymerisation]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |A [[Polyester]] is formed along with [[Water]]. | | style="height:20px; width:200px; text-align:center;" |A [[Polyester]] is formed along with [[Water]]. | ||
| Line 60: | Line 60: | ||
|[[File:StructuralDiagramStarch.png|center|200px]] | |[[File:StructuralDiagramStarch.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Glucose]] [[molecule]]s [[Chemical Reaction|react]] together in a | + | | style="height:20px; width:200px; text-align:center;" |[[Glucose]] [[molecule]]s [[Chemical Reaction|react]] together in a [[Condensation Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Starch]] is formed along with [[Water]]. | | style="height:20px; width:200px; text-align:center;" |[[Starch]] is formed along with [[Water]]. | ||
| Line 71: | Line 71: | ||
|[[File:StructuralDiagramPolyglycine.png|center|200px]] | |[[File:StructuralDiagramPolyglycine.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Glycine]] [[molecule]]s [[Chemical Reaction|react]] together in a | + | | style="height:20px; width:200px; text-align:center;" |[[Glycine]] [[molecule]]s [[Chemical Reaction|react]] together in a [[Condensation Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |A [[Polypeptide]] ([[Protein]]) is formed along with [[Water]]. In reality [[Polypeptide]]s are made of many different [[Peptide]]s ([[Amino Acid]]s) rather than the same one repeated. | | style="height:20px; width:200px; text-align:center;" |A [[Polypeptide]] ([[Protein]]) is formed along with [[Water]]. In reality [[Polypeptide]]s are made of many different [[Peptide]]s ([[Amino Acid]]s) rather than the same one repeated. | ||
|} | |} | ||
Revision as of 15:25, 19 January 2019
Key Stage 4
Meaning
Polymerisation is a chemical reaction in which small molecules known as monomers react to form a polymer.
About Polymerisation
Polymerisation may happen between:
- Identical monomers - Alkenes to Polyalkenes
- Two different monomers with complimantary functional groups at each end. - Esters to Polyesters
- Several different monomers of a homologous series - Peptides to Polypeptides.
Examples
| Ethene monomers can react together in an Addition Polymerisation reaction. | Polythene (sometimes spelled Polyethene) is formed. |
| Tetrafluoroethene monomers can react together in an Addition Polymerisation reaction. | Polytetrafluoroethene (sometimes referred to as PTFE or by a trademark TeflonTM) is formed. |
| Propene monomers can react together in an Addition Polymerisation reaction. | Polypropene is formed. |
| Ethandioate and Ethandiol can react together in a Condensation Polymerisation. | A Polyester is formed along with Water. |
| Glucose molecules react together in a Condensation Polymerisation reaction. | Starch is formed along with Water. |
| Glycine molecules react together in a Condensation Polymerisation reaction. | A Polypeptide (Protein) is formed along with Water. In reality Polypeptides are made of many different Peptides (Amino Acids) rather than the same one repeated. |