Difference between revisions of "Condensation Polymerisation"
(→Forming Polysaccharides) |
|||
| Line 9: | Line 9: | ||
: '''Condensation polymerisation''' [[Chemical Reaction|reactions]] which [[product|produce]] [[Water]] can happen when one end of a [[molecule]] carries an -OH [[Functional Group|group]] while the other end carries a -COOH [[Functional Group|group]]. These [[Chemical Reaction|react]] together joining the [[molecule]]s forming a [[Polyester]]. | : '''Condensation polymerisation''' [[Chemical Reaction|reactions]] which [[product|produce]] [[Water]] can happen when one end of a [[molecule]] carries an -OH [[Functional Group|group]] while the other end carries a -COOH [[Functional Group|group]]. These [[Chemical Reaction|react]] together joining the [[molecule]]s forming a [[Polyester]]. | ||
: '''Condensation polymerisation''' [[Chemical Reaction|reactions]] which [[product|produce]] [[Water]] can also happen when one [[molecule]] carries two -OH [[Functional Group|groups]] at either end of the [[molecule]] while the other [[molecule]] carries two -COOH [[Functional Group|groups]]. These two [[molecule]]s [[Chemical Reaction|react]] together joining the [[molecule]]s forming a [[Polyester]] and [[product|producing]] [[Water]]. | : '''Condensation polymerisation''' [[Chemical Reaction|reactions]] which [[product|produce]] [[Water]] can also happen when one [[molecule]] carries two -OH [[Functional Group|groups]] at either end of the [[molecule]] while the other [[molecule]] carries two -COOH [[Functional Group|groups]]. These two [[molecule]]s [[Chemical Reaction|react]] together joining the [[molecule]]s forming a [[Polyester]] and [[product|producing]] [[Water]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:StructuralDiagramEthandioateEthandiol.png|center|200px]] | ||
| + | |[[File:ArrowRight.png|center|200px]] | ||
| + | |[[File:StructuralDiagramPolyester.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | |} | ||
====Forming Polysaccharides==== | ====Forming Polysaccharides==== | ||
| Line 18: | Line 28: | ||
Where 'n' represents an [[integer]]. | Where 'n' represents an [[integer]]. | ||
| − | + | {| class="wikitable" | |
| + | |- | ||
| + | |[[File:StructuralDiagramGlucose.png|center|200px]] | ||
| + | |[[File:ArrowRight.png|center|200px]] | ||
| + | |[[File:StructuralDiagramStarch.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | |} | ||
====Forming Proteins==== | ====Forming Proteins==== | ||
: '''Condensation polymerisation''' [[Chemical Reaction|reactions]] of [[Peptide]]s ([[Amino Acid]]s) [[product|produce]] [[Polypeptide]]s ([[Protien]]s) and [[Water]]. | : '''Condensation polymerisation''' [[Chemical Reaction|reactions]] of [[Peptide]]s ([[Amino Acid]]s) [[product|produce]] [[Polypeptide]]s ([[Protien]]s) and [[Water]]. | ||
| Line 27: | Line 46: | ||
Where 'n' represents an [[integer]]. | Where 'n' represents an [[integer]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:StructuralDiagramGlycine.png|center|200px]] | ||
| + | |[[File:ArrowRight.png|center|200px]] | ||
| + | |[[File:StructuralDiagramPolyglycine.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | |} | ||
Revision as of 13:50, 19 January 2019
Contents
Key Stage 4 Higher
Meaning
Condensation polymerisation is a reaction in which monomers combine to form polymers producing a H2O or HCl molecule for each addition of a monomer.
About Condensation Polymerisation
- Condensation polymerisation reactions are called condensation reactions because they usually produce Water, but they may also produce other small molecules such as HCl.
- Condensation polymerisation reactions happen commonly in biological organisms.
Forming Polyesters
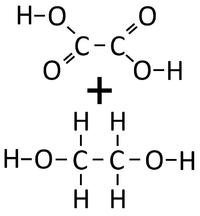
- Condensation polymerisation reactions which produce Water can happen when one end of a molecule carries an -OH group while the other end carries a -COOH group. These react together joining the molecules forming a Polyester.
- Condensation polymerisation reactions which produce Water can also happen when one molecule carries two -OH groups at either end of the molecule while the other molecule carries two -COOH groups. These two molecules react together joining the molecules forming a Polyester and producing Water.
| Text | Text | Text |
Forming Polysaccharides
- The condensation polymerisation reaction of monosaccharides produces a polysaccharide and Water.
- The monosaccharide glucose can bond in a condensation polymersiation reaction to produce starch or glycogen.
n x Monosaccharides → Polysaccharide + Water
n x Glucose → Starch + Water
Where 'n' represents an integer.
| Text | Text | Text |
Forming Proteins
- Condensation polymerisation reactions of Peptides (Amino Acids) produce Polypeptides (Protiens) and Water.
- Each Amino Acid has an NH2 group which acts as a base and an -COOH group which acts as an acid. These functional groups react to produce Polypeptides and Water.
n x Peptides → Polypeptide + Water
<chem>nH2NCH2COOH -> (-HNCH2CO-) + nH2O</chem>
Where 'n' represents an integer.
| Text | Text | Text |