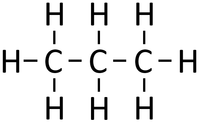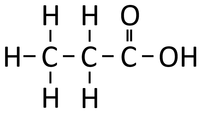Difference between revisions of "Functional Group"
(Created page with "==Key Stage 4== ===Meaning=== A '''functional group''' is a group of atoms responsible for the chemical properties of a compound. ===About Funct...") |
|||
| Line 9: | Line 9: | ||
*[[Alcohol]]s - The '''functional group''' is the OH group attached to a [[Carbon]] [[atom]]. | *[[Alcohol]]s - The '''functional group''' is the OH group attached to a [[Carbon]] [[atom]]. | ||
*[[Carboxylic Acid]]s - The '''functional group''' is the [[Double Bond|double bond]] between a [[Carbon]] [[atom]] and an [[Oxygen]] [[atom]] and the [[Single Bond|single bond]] to an OH group on that same [[Carbon]] [[atom]]. | *[[Carboxylic Acid]]s - The '''functional group''' is the [[Double Bond|double bond]] between a [[Carbon]] [[atom]] and an [[Oxygen]] [[atom]] and the [[Single Bond|single bond]] to an OH group on that same [[Carbon]] [[atom]]. | ||
| + | |||
| + | ===Examples=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Propane''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Propene''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Propanol''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Propanoic Acid''' | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Chemical Formula]] | ||
| + | | style="height:20px; width:200px; text-align:center;" |C<sub>3</sub>H<sub>8</sub> | ||
| + | | style="height:20px; width:200px; text-align:center;" |C<sub>3</sub>H<sub>6</sub> | ||
| + | | style="height:20px; width:200px; text-align:center;" |C<sub>3</sub>H<sub>8</sub>O | ||
| + | | style="height:20px; width:200px; text-align:center;" |C<sub>3</sub>H<sub>6</sub>O<sub>2</sub> | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Structural Formula]] | ||
| + | | style="height:20px; width:200px; text-align:center;" |CH<sub>3</sub>CH<sub>2</sub>CH<sub>3</sub> | ||
| + | | style="height:20px; width:200px; text-align:center;" |CH<sub>2</sub>CHCH<sub>3</sub> | ||
| + | | style="height:20px; width:200px; text-align:center;" |CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH | ||
| + | | style="height:20px; width:200px; text-align:center;" |CH<sub>3</sub>CH<sub>2</sub>COOH | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Structural Diagram]] | ||
| + | |[[File:StructuralDiagramPropane.png|center|200px]] | ||
| + | |[[File:StructuralDiagramPropene.png|center|200px]] | ||
| + | |[[File:StructuralDiagramPropanol.png|center|200px]] | ||
| + | |[[File:StructuralDiagramPropanoicAcid.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Ball and Stick Model]] | ||
| + | |[[File:BallandStickPropane.png|center|200px]] | ||
| + | |[[File:BallandStickPropene.png|center|200px]] | ||
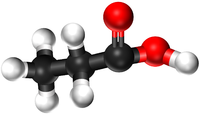
| + | |[[File:BallandStickPropanol.png|center|200px]] | ||
| + | |[[File:BallandStickPropanoicAcid.png|center|200px]] | ||
| + | |} | ||
Revision as of 16:44, 17 January 2019
Key Stage 4
Meaning
A functional group is a group of atoms responsible for the chemical properties of a compound.
About Functional Groups
There are four important functional groups you should know:
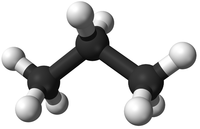
- Alkanes - The functional group is the single bonds between Carbon atoms and between Carbon and Hydrogen atoms.
- Alkenes - The functional group is the double bonds between Carbon atoms.
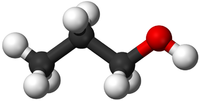
- Alcohols - The functional group is the OH group attached to a Carbon atom.
- Carboxylic Acids - The functional group is the double bond between a Carbon atom and an Oxygen atom and the single bond to an OH group on that same Carbon atom.
Examples
| Propane | Propene | Propanol | Propanoic Acid | |
| Chemical Formula | C3H8 | C3H6 | C3H8O | C3H6O2 |
| Structural Formula | CH3CH2CH3 | CH2CHCH3 | CH3CH2CH2OH | CH3CH2COOH |
| Structural Diagram | ||||
| Ball and Stick Model |