Difference between revisions of "Indicator (Chemistry)"
| Line 23: | Line 23: | ||
===Examples=== | ===Examples=== | ||
| + | {| class="wikitable" | ||
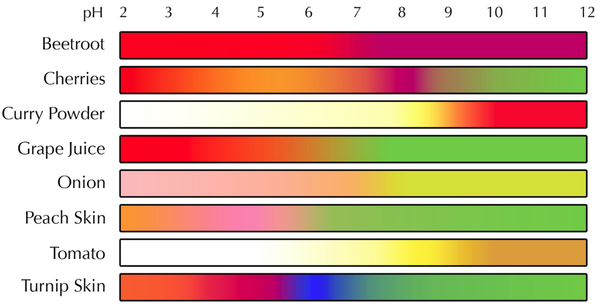
| + | |+ These are the colour ranges of different '''indicator''' plants. | ||
| + | |- | ||
| + | |[[File:NaturalIndicators.png|center|600px]] | ||
| + | |- | ||
| + | |} | ||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | An '''indicator''' is a [[dye]] that changes [[colour]] when other [[chemical]]s are present. | ||
| + | |||
| + | ===About Indicators=== | ||
| + | : Some [[indicator]]s detect [[pH]] but others can be used to detect other [[chemical]]s. | ||
| + | Some non-pH '''indicators''' you should know: | ||
| + | *[[Iodine Solution]] - Used to detect starch. | ||
| + | *[[Biuret Solution]] - Used to detect proteins. | ||
| + | *[[Benedict's Test|Benedict's Solution]] - Used to detect simple [[sugar]]s like [[glucose]]. | ||
| + | *[[Limewater]] - Used to detect [[Carbon Dioxide]]. | ||
| + | ===About pH Indicators=== | ||
| + | : The [[colour]] of a '''pH indicator''' can be used to tell the [[pH]] of a [[solution]]. | ||
| + | : Different '''indicators''' will have a different range of colours for different [[pH]] values. | ||
| + | : A good '''indicator''' can be added to [[solution]] without affecting the [[pH]] of the [[solution]]. If an '''indicator''' change the [[pH]] of a [[solution]] it could not give an [[accurate]] [[reading]]. | ||
| + | Some '''pH indicators''' you should know: | ||
| + | *[[Litmus Paper]] | ||
| + | *[[Red Cabbage Indicator]] | ||
| + | *[[Universal Indicator]] | ||
| + | *[[Phenolphthalein]] | ||
| + | *[[Methyl Orange]] | ||
| + | *[[Bromothymol Blue]] | ||
| + | |||
| + | ===Examples=== | ||
{| class="wikitable" | {| class="wikitable" | ||
|+ These are the colour ranges of different '''indicator''' plants. | |+ These are the colour ranges of different '''indicator''' plants. | ||
Revision as of 21:23, 30 March 2019
Contents
Key Stage 3
Meaning
An indicator is a dye that changes colour when other chemicals are present.
About Indicators
- Some indicators detect pH but others can be used to detect other chemicals.
Some non-pH indicators you should know:
- Iodine Solution - Used to detect starch.
- Biuret Solution - Used to detect proteins.
- Benedict's Solution - Used to detect simple sugars like glucose.
- Limewater - Used to detect Carbon Dioxide.
About pH Indicators
- The colour of a pH indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good indicator can be added to solution without affecting the pH of the solution. If an indicator change the pH of a solution it could not give an accurate reading.
Some pH indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue
Examples
Key Stage 4
Meaning
An indicator is a dye that changes colour when other chemicals are present.
About Indicators
- Some indicators detect pH but others can be used to detect other chemicals.
Some non-pH indicators you should know:
- Iodine Solution - Used to detect starch.
- Biuret Solution - Used to detect proteins.
- Benedict's Solution - Used to detect simple sugars like glucose.
- Limewater - Used to detect Carbon Dioxide.
About pH Indicators
- The colour of a pH indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good indicator can be added to solution without affecting the pH of the solution. If an indicator change the pH of a solution it could not give an accurate reading.
Some pH indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue