Difference between revisions of "Isotope"
| Line 6: | Line 6: | ||
: Each [[element]] has many [[isotope]]s but some are [[Stable Isotope|stable]] and others are [[Unstable Isotope|unstable]] so they [[Radioactive Decay|decay]] quickly into other [[isotope]]s or a different [[element]]. | : Each [[element]] has many [[isotope]]s but some are [[Stable Isotope|stable]] and others are [[Unstable Isotope|unstable]] so they [[Radioactive Decay|decay]] quickly into other [[isotope]]s or a different [[element]]. | ||
: Different [[isotope]]s of the same [[element]] have the same [[Atomic Number|atomic number]] but different [[Relative Atomic Mass|atomic mass]] due to the different numbers of [[neutron]]s. | : Different [[isotope]]s of the same [[element]] have the same [[Atomic Number|atomic number]] but different [[Relative Atomic Mass|atomic mass]] due to the different numbers of [[neutron]]s. | ||
| + | |||
| + | ===Examples=== | ||
{| class="wikitable" | {| class="wikitable" | ||
| style="height:20px; width:200px; text-align:center;" |'''Hydrogen-1''' | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen-1''' | ||
Revision as of 00:16, 26 November 2018
Key Stage 4
Meaning
Isotopes are atoms with the same number of protons (the same element) but a different number of neutrons.
About Isotopes
- Each element has many isotopes but some are stable and others are unstable so they decay quickly into other isotopes or a different element.
- Different isotopes of the same element have the same atomic number but different atomic mass due to the different numbers of neutrons.
Examples
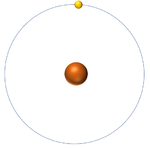
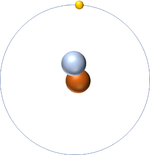
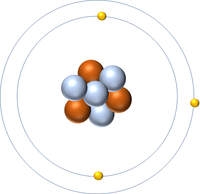
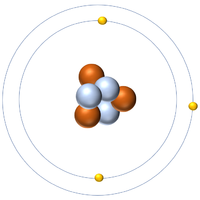
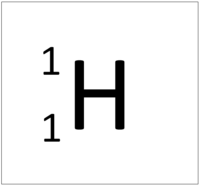
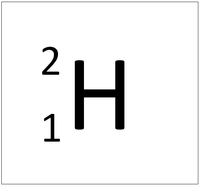
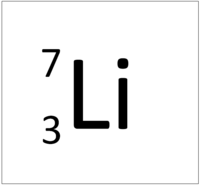
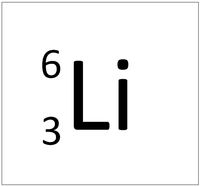
| Hydrogen-1 | Hydrogen-2 | Lithium-7 | Lithium-6 |
| Hydrogen always has 1 proton but in this case has no neutrons. | Hydrogen always has 1 proton but in this case also has a neutron. This isotope of Hydrogen is known as Deuterium. | Lithium always has 3 protons but in this case has 4 neutrons. | Lithium always has 3 protons but in this case has 3 neutrons. |