Difference between revisions of "Plum Pudding Model"
(Created page with "==Key Stage 4== ===Meaning=== right|300px|thumb|A diagram of an [[atom in the Plum Pudding Model.]] The Plum Pudding Model is a model o...") |
|||
| Line 6: | Line 6: | ||
===About the Plum Pudding Model=== | ===About the Plum Pudding Model=== | ||
: In the [[Plum Pudding Model]] the [[atom]] is [[Neutral Charge|neutral]] because the [[Negative Charge|negatively charged]] [[electron]]s are fixed within a larger sphere of [[Positive Charge|positive charge]]. | : In the [[Plum Pudding Model]] the [[atom]] is [[Neutral Charge|neutral]] because the [[Negative Charge|negatively charged]] [[electron]]s are fixed within a larger sphere of [[Positive Charge|positive charge]]. | ||
| + | : The [[Plum Pudding Model]] is named after a desert made from sponge with plums stuck inside. It was imagined that the [[atom]] had a [[sphere]] of [[Positive Charge|positive charge]] like the sponge of the cake and [[electron]]s suck inside, like the plums stuck inside the sponge. | ||
: The [[Plum Pudding Model]] was proposed by [[J.J. Thompson]] who discovered the [[electron]] and realised it was part of an [[atom]]. | : The [[Plum Pudding Model]] was proposed by [[J.J. Thompson]] who discovered the [[electron]] and realised it was part of an [[atom]]. | ||
: The [[Plum Pudding Model]] was proven false by [[Rutherford's Alpha Scattering Experiment]] and was replaced by the [[Nuclear Model]]. | : The [[Plum Pudding Model]] was proven false by [[Rutherford's Alpha Scattering Experiment]] and was replaced by the [[Nuclear Model]]. | ||
Revision as of 12:00, 24 November 2018
Key Stage 4
Meaning
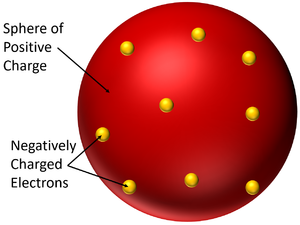
The Plum Pudding Model is a model of the atom which suggests the atom is a solid sphere of positive charge with negatively charged electrons spread within it.
About the Plum Pudding Model
- In the Plum Pudding Model the atom is neutral because the negatively charged electrons are fixed within a larger sphere of positive charge.
- The Plum Pudding Model is named after a desert made from sponge with plums stuck inside. It was imagined that the atom had a sphere of positive charge like the sponge of the cake and electrons suck inside, like the plums stuck inside the sponge.
- The Plum Pudding Model was proposed by J.J. Thompson who discovered the electron and realised it was part of an atom.
- The Plum Pudding Model was proven false by Rutherford's Alpha Scattering Experiment and was replaced by the Nuclear Model.