Difference between revisions of "Distillation"
| Line 11: | Line 11: | ||
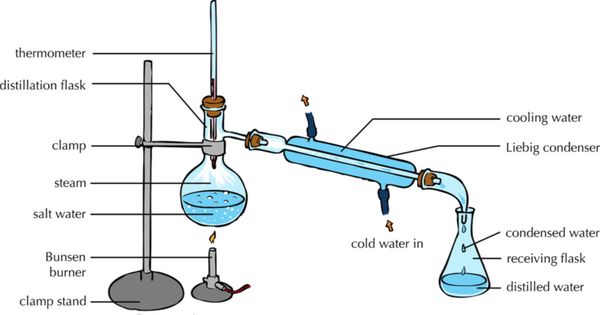
| style="height:20px; width:600px; text-align:center;" |This [[diagram]] shows how the [[solute]] and [[solvent]] can be [[Separating Mixtures|separated]] by heating the [[solution]] to [[evaporate]] the [[solvent]] leaving the [[solute]] behind and then [[condensing]] the [[evaporate]]d [[solvent]] by cooling it down and collecting it in a [[Conical Flask|conical flask]]. | | style="height:20px; width:600px; text-align:center;" |This [[diagram]] shows how the [[solute]] and [[solvent]] can be [[Separating Mixtures|separated]] by heating the [[solution]] to [[evaporate]] the [[solvent]] leaving the [[solute]] behind and then [[condensing]] the [[evaporate]]d [[solvent]] by cooling it down and collecting it in a [[Conical Flask|conical flask]]. | ||
|} | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Distillation]] is a technique used to [[Separating Mixtures|separate]] [[solute]]s and [[solvent]]s from [[solution]]] that can recover both the [[solute]] and the [[solvent]]. | ||
| + | |||
| + | ===About Distillation=== | ||
Revision as of 16:40, 20 January 2019
Contents
Key Stage 3
Meaning
Distillation is a method of separating mixtures that can recover both the solute and the solvent in a solution.
About Distillation
- During distillation the solvent is evaporated away to leave the solute behind, then the solvent is collected and condensed back into a liquid.
| This diagram shows how the solute and solvent can be separated by heating the solution to evaporate the solvent leaving the solute behind and then condensing the evaporated solvent by cooling it down and collecting it in a conical flask. |
Key Stage 4
Meaning
Distillation is a technique used to separate solutes and solvents from solution] that can recover both the solute and the solvent.