Difference between revisions of "Evaporation of Solutions"
(→Meaning) |
|||
| Line 33: | Line 33: | ||
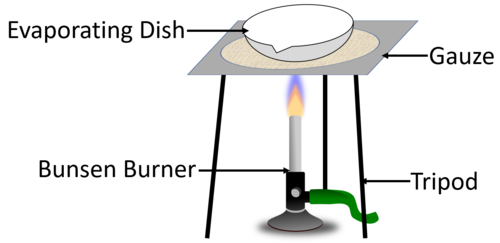
| style="height:20px; width:200px; text-align:center;" |The [[diagram]] shows the experimental setup to [[evaporating|evaporate]] away the [[solvent]] to leave behind the [[solute]] in the [[Evaporating Dish|evaporating dish]]. | | style="height:20px; width:200px; text-align:center;" |The [[diagram]] shows the experimental setup to [[evaporating|evaporate]] away the [[solvent]] to leave behind the [[solute]] in the [[Evaporating Dish|evaporating dish]]. | ||
|} | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | '''Evaporation of solution''', sometimes called '''Crystallisation''' is a technique used to [[Separating Mixtures|separate]] the [[solute]] from the [[solvent]] in a [[solution]], losing the [[solvent]]. | ||
| + | |||
| + | ===About Evaporation of Solutions=== | ||
Revision as of 16:17, 20 January 2019
Contents
Key Stage 2
Meaning
Evaporation of a solution is a way to get back a solid that has been dissolved in a liquid.
About Evaporation of Solutions
| You can separate salt from the water by evaporating the water in an evaporating dish. |
Examples
| These people collect salt by putting sea water in small ponds and allowing the warm temperatures to evaporate the water away leaving the behind the salt. |
Key Stage 3
Meaning
Evaporation of a solution is a way to separate the mixture of a solution to recover the solute that has been dissolved in a solvent.
About Evaporation of Solutions
- The evaporation of solutions recovers the solutes but loses the solvent.
- Evaporation of solutions can be done by directly heating the solution or by giving time for the liquid to evaporate at low temperatures.
| The diagram shows the experimental setup to evaporate away the solvent to leave behind the solute in the evaporating dish. |
Key Stage 4
Meaning
Evaporation of solution, sometimes called Crystallisation is a technique used to separate the solute from the solvent in a solution, losing the solvent.