Difference between revisions of "Chemical Reaction"
(→Types of Chemical Reaction) |
(→Types of Chemical Reaction) |
||
| Line 49: | Line 49: | ||
*[[Combustion]] - A [[chemical]] burns in the presence of [[Oxygen]]. | *[[Combustion]] - A [[chemical]] burns in the presence of [[Oxygen]]. | ||
*[[Thermal Decomposition]] - A [[compound]] breaks down into smaller [[compound]]s. | *[[Thermal Decomposition]] - A [[compound]] breaks down into smaller [[compound]]s. | ||
| − | *[[Oxidation]] - A [[chemical]] reacts with [[Oxygen]]. | + | *[[Oxidation]] - A [[chemical]] '''reacts''' with [[Oxygen]]. |
*[[Displacement Reaction]] - One [[element]] replaces another in a [[compound]]. | *[[Displacement Reaction]] - One [[element]] replaces another in a [[compound]]. | ||
*[[Neutralisation]] - An [[acid]] and a [[base]] '''react''' to make a [[salt]]. | *[[Neutralisation]] - An [[acid]] and a [[base]] '''react''' to make a [[salt]]. | ||
Revision as of 19:15, 23 September 2018
Contents
Key Stage 2
Meaning
A chemical reaction is when one substance changes into another.
About Chemical Reactions
- Most chemical reactions are irreversible.
- Chemical reactions usually cause a change in temperature.
- Chemical reactions you should know are:
Key Stage 3
Meaning
A Chemical Reaction is when the atoms in molecules break their bonds and form new bonds, usually with other atoms.
About Chemical Reactions
- Most chemical reactions are irreversible.
- In a chemical reaction the atoms rearrange to form a new substance.
- Chemical reactions often need some energy to start, like combustion where the fuel needs to be heated to start.
- If a chemical reaction gives off more energy than it needs to start then it is called an Exothermic Reaction. In these reactions the temperature increases.
- If a chemical reaction takes in more energy than it gives out then it is called an Endothermic Reaction.
In these reactions the temperature decreases.
Examples
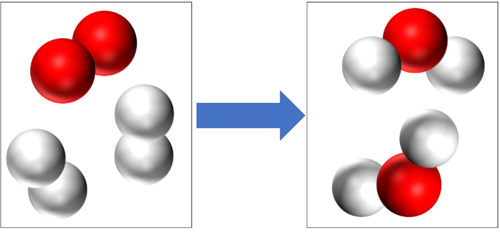
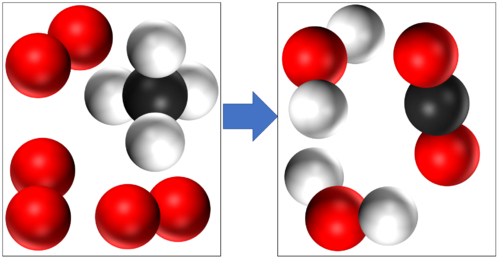
These are two examples of Combustion
|
2H2 + O2 → 2H2O |
|
Methane + Oxygen → Water + Carbon Dioxide CH4 + 2O2 → 2H2O + CO2 |
Types of Chemical Reaction
- Combustion - A chemical burns in the presence of Oxygen.
- Thermal Decomposition - A compound breaks down into smaller compounds.
- Oxidation - A chemical reacts with Oxygen.
- Displacement Reaction - One element replaces another in a compound.
- Neutralisation - An acid and a base react to make a salt.