Difference between revisions of "PH"
| Line 1: | Line 1: | ||
==Key Stage 3== | ==Key Stage 3== | ||
| + | [[File:pHScale.png|right|300px|thumb|A '''pH''' scale with the colours of [[Universal Indicator]] at those '''pH''' values and some examples of [[substance]]s at those '''pH''' values.]] | ||
===Meaning=== | ===Meaning=== | ||
| − | |||
The '''pH''' scale is a numbered list from 0 to 14 that is used to identify how [[Acid|acidic]] or [[Base|basic]] a [[substance]] is. | The '''pH''' scale is a numbered list from 0 to 14 that is used to identify how [[Acid|acidic]] or [[Base|basic]] a [[substance]] is. | ||
Revision as of 17:13, 28 June 2019
Contents
Key Stage 3
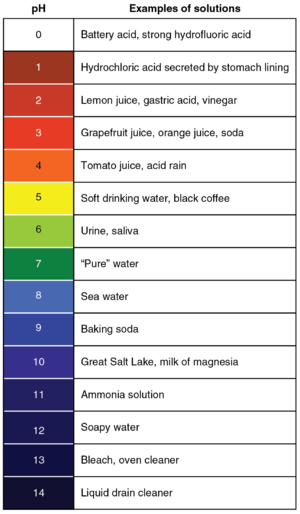
A pH scale with the colours of Universal Indicator at those pH values and some examples of substances at those pH values.
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About pH
- pH is written with a lower case p and an upper case H and refers to the 'power of Hydrogen' as there are free Hydrogen ions in an acid.
- Acids have a pH of less than 7 with the strongest acids being at pH 0.
- Bases have a pH greater than 7 with the strongest bases being at pH 14. Remember alkalis are a base dissolved in water.
- pH 7.0 is neutral.
Key Stage 4 Foundation
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About the pH Scale
- The pH of a solution is determined by the strength and concentration of Hydrogen ions in an acid or Hydroxide ions in an alkali.
- An acid will have a greater concentration of Hydrogen ions the lower on the pH scale.
- An alkali will have a greater concentration of Hydroxide ions the higher on the pH scale.
- A neutral solution will have an equal concentration of Hydrogen ions and Hydroxide ions and have a pH of 7.
Key Stage 4 Higher
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About the pH Scale
- The pH of a solution is determined by the strength and concentration of Hydrogen ions in an acid or Hydroxide ions in an alkali.
- An acid will have a greater concentration of Hydrogen ions the lower on the pH scale.
- An alkali will have a greater concentration of Hydroxide ions the higher on the pH scale.
- A neutral solution will have an equal concentration of Hydrogen ions and Hydroxide ions and have a pH of 7.
- In acids for a pH decrease of 1 there must be 10 times greater concentration of Hydrogen ions in solution.