Difference between revisions of "Methanol"
(→About Methanol) |
|||
| Line 30: | Line 30: | ||
: The [[combustion]] of [[methanol]] [[product|produces]] [[Carbon Dioxide]] and [[Water]]. | : The [[combustion]] of [[methanol]] [[product|produces]] [[Carbon Dioxide]] and [[Water]]. | ||
: [[Methanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | : [[Methanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| − | : < | + | : <math>2CH_3OH + 3O_2 → 2CO_2 + 4H_2O</math> |
: [[Methanol]] can also be [[oxidise]]d at low [[temperature]]s to [[product|produce]] [[Water]] and the [[Carboxylic Acid]]; [[Methanoic Acid]]. | : [[Methanol]] can also be [[oxidise]]d at low [[temperature]]s to [[product|produce]] [[Water]] and the [[Carboxylic Acid]]; [[Methanoic Acid]]. | ||
| − | : < | + | : <math>CH_3OH + O_2 → CHOOH + H_2O</math> |
Revision as of 14:17, 7 June 2019
Key Stage 3
Meaning
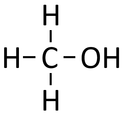
Methanol is a compound with chemical formula CH3OH.
About Methanol
- Methanol is a transparent liquid (at room temperature).
- Methanol can be oxidised to produce Carbon Dioxide and Water.
- Methanol + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Methanol is an alcohol molecule with chemical formula CH3OH.
About Methanol
- Methanol is a transparent liquid (at STP).
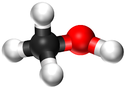
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| CH3OH | CH3OH |
- The combustion of methanol produces Carbon Dioxide and Water.
- Methanol + Oxygen → Carbon Dioxide + Water
\[2CH_3OH + 3O_2 → 2CO_2 + 4H_2O\]
- Methanol can also be oxidised at low temperatures to produce Water and the Carboxylic Acid; Methanoic Acid.
\[CH_3OH + O_2 → CHOOH + H_2O\]