Difference between revisions of "GCSE Physics Required Practical: Determining Specific Heat Capacity"
(→Method) |
|||
| Line 23: | Line 23: | ||
#Switch on the [[Power Supply|power supply]]. | #Switch on the [[Power Supply|power supply]]. | ||
#Record the [[reading]] on the [[Joulemeter]] with every 2°C increase in [[temperature]] a minimum of 6 times. | #Record the [[reading]] on the [[Joulemeter]] with every 2°C increase in [[temperature]] a minimum of 6 times. | ||
| + | #Plot a [[graph]] with [[energy]] on the [[y-axis]] and [[temperature]] on the [[x-axis]]. | ||
| + | : Given the equation <math>E_T=mc \Delta \theta</math> then the [[gradient]] of this graph will be the [[mass]] multiplied by the [[Specific Heat Capacity|specific heat capacity]] (mc). | ||
| + | |||
| + | ====Improving [[Accuracy]]==== | ||
| + | : Place the [[metal]] block on a [[Heatproof Mat|heatproof mat]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the table surface by [[Thermal Conduction|conduction]]. | ||
| + | : Wrap the [[metal]] block a [[Thermal Insulator|thermal insulator]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the [[air]]. | ||
| + | : Complete the [[experiment]] in [[temperature]] range close to [[Room Temperature|room temperature]] to reduce the rate of [[Energy Transfer|energy transfer]] from the [[metal]] block to the surroundings. | ||
| + | |||
| + | ====Improving [[Precision]]==== | ||
| + | : Use a [[thermometer]] with a higher [[resolution]]. | ||
| + | : Use a [[Data Logger|data logger]] rather than a [[thermometer]]. | ||
| + | |||
| + | ===Experiment Version 1b=== | ||
| + | ====Variables==== | ||
| + | : [[Independent Variable]]: The [[energy]] supplied to the [[metal]] block by [[heating]]. | ||
| + | : [[Dependent Variable]]: The [[temperature]] of the [[metal]] block. | ||
| + | : [[Control Variable]]s: The [[mass]] of the [[metal]] block. | ||
| + | |||
| + | ====Method==== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RequiredPracticalSHC1.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the [[apparatus]] used in an [[experiment]] to find the [[Specific Heat Capacity|specific heat capacity]] of a [[metal]] block. | ||
| + | |} | ||
| + | |||
| + | #Attach a [[Joulemeter]] and [[Power Supply|power supply]] to an [[Immersion Heater|immersion heater]]. | ||
| + | #Place the [[Immersion Heater|immersion heater]] and the [[thermometer]] in holes in the [[metal]] block. | ||
| + | #Place a drop of [[water]] in the [[thermometer]] hole to ensure [[Thermal Contact|thermal contact]] between the [[thermometer]] and the [[metal]] block. | ||
| + | #[[Reading|Read]] and record the initial [[temperature]] of the [[metal]] block. | ||
| + | #Switch on the [[Power Supply|power supply]]. | ||
| + | #Record the [[reading]] on the [[thermometer]] with every 1000J shown on the [[joulemeter]] a minimum of 6 times. | ||
| + | #Plot a [[graph]] with [[energy]] on the [[y-axis]] and [[temperature]] on the [[x-axis]]. | ||
| + | : Given the equation <math>E_T=mc \Delta \theta</math> then the [[gradient]] of this graph will be the [[mass]] multiplied by the [[Specific Heat Capacity|specific heat capacity]] (mc). | ||
| + | |||
| + | ====Improving [[Accuracy]]==== | ||
| + | : Place the [[metal]] block on a [[Heatproof Mat|heatproof mat]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the table surface by [[Thermal Conduction|conduction]]. | ||
| + | : Wrap the [[metal]] block a [[Thermal Insulator|thermal insulator]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the [[air]]. | ||
| + | : Complete the [[experiment]] in [[temperature]] range close to [[Room Temperature|room temperature]] to reduce the rate of [[Energy Transfer|energy transfer]] from the [[metal]] block to the surroundings. | ||
| + | |||
| + | ====Improving [[Precision]]==== | ||
| + | : Use a [[thermometer]] with a higher [[resolution]]. | ||
| + | : Use a [[Data Logger|data logger]] rather than a [[thermometer]]. | ||
| + | |||
| + | ===Experiment Version 2a=== | ||
| + | ====Variables==== | ||
| + | : [[Independent Variable]]: The [[temperature]] of the [[metal]] block. | ||
| + | : [[Dependent Variable]]: The [[time]] over which [[energy]] is supplied to the [[metal]] block. | ||
| + | : [[Control Variable]]s: The [[mass]] of the [[metal]] block. The [[power]] of the [[Immersion Heater|immersion heater]]. | ||
| + | |||
| + | ====Method==== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RequiredPracticalSHC2.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the [[apparatus]] used in an [[experiment]] to find the [[Specific Heat Capacity|specific heat capacity]] of a [[metal]] block. | ||
| + | |} | ||
| + | |||
| + | #Connect an [[Ammeter]], [[Power Supply|power supply]] and [[Immersion Heater|immersion heater]] in [[Series Circuit|series]]. | ||
| + | #Connect a [[voltmeter]] in [[Parallel Circuit|parallel]] to the [[Immersion Heater|immersion heater]]. | ||
| + | #Place the [[Immersion Heater|immersion heater]] and the [[thermometer]] in holes in the [[metal]] block. | ||
| + | #Place a drop of [[water]] in the [[thermometer]] hole to ensure [[Thermal Contact|thermal contact]] between the [[thermometer]] and the [[metal]] block. | ||
| + | #[[Reading|Read]] and record the initial [[temperature]] of the [[metal]] block. | ||
| + | #Switch on the [[Power Supply|power supply]], start a [[stopwatch]] and record the [[reading]]s on the [[Voltmeter]] and [[Ammeter]].#Record the [[time]] on the [[stopwatch]] with every 2°C increase in [[temperature]] a minimum of 6 times. | ||
| + | #Use the equation <math>E = IVt</math> to calculate the [[energy]] supplied to the [[metal]] block. | ||
| + | #Plot a [[graph]] with [[energy]] on the [[y-axis]] and [[temperature]] on the [[x-axis]]. | ||
| + | : Given the equation <math>E_T=mc \Delta \theta</math> then the [[gradient]] of this graph will be the [[mass]] multiplied by the [[Specific Heat Capacity|specific heat capacity]] (mc). | ||
| + | |||
| + | ====Improving [[Accuracy]]==== | ||
| + | : Place the [[metal]] block on a [[Heatproof Mat|heatproof mat]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the table surface by [[Thermal Conduction|conduction]]. | ||
| + | : Wrap the [[metal]] block a [[Thermal Insulator|thermal insulator]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the [[air]]. | ||
| + | : Complete the [[experiment]] in [[temperature]] range close to [[Room Temperature|room temperature]] to reduce the rate of [[Energy Transfer|energy transfer]] from the [[metal]] block to the surroundings. | ||
| + | |||
| + | ====Improving [[Precision]]==== | ||
| + | : Use a [[thermometer]] with a higher [[resolution]]. | ||
| + | : Use a [[Data Logger|data logger]] rather than a [[thermometer]]. | ||
| + | |||
| + | ===Experiment Version 2a=== | ||
| + | ====Variables==== | ||
| + | : [[Independent Variable]]: The [[time]] over which [[energy]] is supplied to the [[metal]] block. | ||
| + | : [[Dependent Variable]]: The [[temperature]] of the [[metal]] block. | ||
| + | : [[Control Variable]]s: The [[mass]] of the [[metal]] block. The [[power]] of the [[Immersion Heater|immersion heater]]. | ||
| + | |||
| + | ====Method==== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RequiredPracticalSHC2.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the [[apparatus]] used in an [[experiment]] to find the [[Specific Heat Capacity|specific heat capacity]] of a [[metal]] block. | ||
| + | |} | ||
| + | |||
| + | #Connect an [[Ammeter]], [[Power Supply|power supply]] and [[Immersion Heater|immersion heater]] in [[Series Circuit|series]]. | ||
| + | #Connect a [[voltmeter]] in [[Parallel Circuit|parallel]] to the [[Immersion Heater|immersion heater]]. | ||
| + | #Place the [[Immersion Heater|immersion heater]] and the [[thermometer]] in holes in the [[metal]] block. | ||
| + | #Place a drop of [[water]] in the [[thermometer]] hole to ensure [[Thermal Contact|thermal contact]] between the [[thermometer]] and the [[metal]] block. | ||
| + | #[[Reading|Read]] and record the initial [[temperature]] of the [[metal]] block. | ||
| + | #Switch on the [[Power Supply|power supply]], start a [[stopwatch]] and record the [[reading]]s on the [[Voltmeter]] and [[Ammeter]]. | ||
| + | #[[Reading|Read]] and record the [[temperature]] on the [[thermometer]] every 30 seconds on the [[stopwatch]] a minimum of 6 times. | ||
| + | #Use the equation <math>E = IVt</math> to calculate the [[energy]] supplied to the [[metal]] block. | ||
| + | #Plot a [[graph]] with [[energy]] on the [[y-axis]] and [[temperature]] on the [[x-axis]]. | ||
| + | : Given the equation <math>E_T=mc \Delta \theta</math> then the [[gradient]] of this graph will be the [[mass]] multiplied by the [[Specific Heat Capacity|specific heat capacity]] (mc). | ||
====Improving [[Accuracy]]==== | ====Improving [[Accuracy]]==== | ||
Revision as of 19:40, 18 March 2019
Contents
Key Stage 4
Meaning
Determining the specific heat capacity of a metal block.
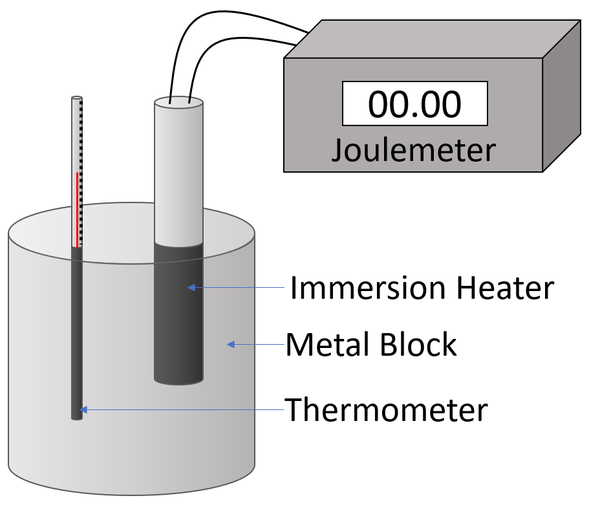
Experiment Version 1a
Variables
- Independent Variable: The temperature of the metal block.
- Dependent Variable: The energy supplied to the metal block by heating.
- Control Variables: The mass of the metal block.
Method
| A diagram of the apparatus used in an experiment to find the specific heat capacity of a metal block. |
- Attach a Joulemeter and power supply to an immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply.
- Record the reading on the Joulemeter with every 2°C increase in temperature a minimum of 6 times.
- Plot a graph with energy on the y-axis and temperature on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of this graph will be the mass multiplied by the specific heat capacity (mc).
Improving Accuracy
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
Improving Precision
- Use a thermometer with a higher resolution.
- Use a data logger rather than a thermometer.
Experiment Version 1b
Variables
- Independent Variable: The energy supplied to the metal block by heating.
- Dependent Variable: The temperature of the metal block.
- Control Variables: The mass of the metal block.
Method
| A diagram of the apparatus used in an experiment to find the specific heat capacity of a metal block. |
- Attach a Joulemeter and power supply to an immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply.
- Record the reading on the thermometer with every 1000J shown on the joulemeter a minimum of 6 times.
- Plot a graph with energy on the y-axis and temperature on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of this graph will be the mass multiplied by the specific heat capacity (mc).
Improving Accuracy
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
Improving Precision
- Use a thermometer with a higher resolution.
- Use a data logger rather than a thermometer.
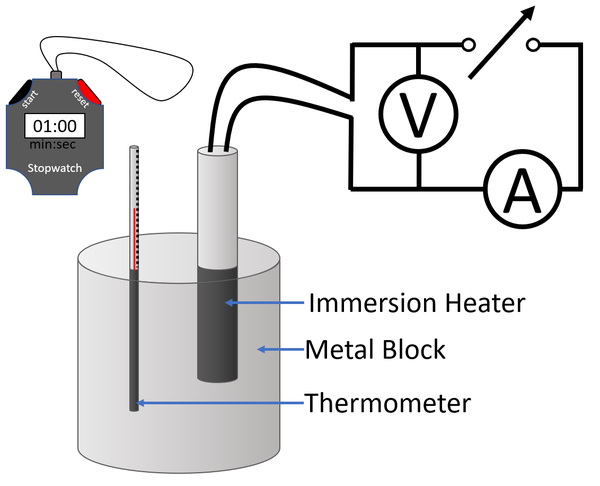
Experiment Version 2a
Variables
- Independent Variable: The temperature of the metal block.
- Dependent Variable: The time over which energy is supplied to the metal block.
- Control Variables: The mass of the metal block. The power of the immersion heater.
Method
| A diagram of the apparatus used in an experiment to find the specific heat capacity of a metal block. |
- Connect an Ammeter, power supply and immersion heater in series.
- Connect a voltmeter in parallel to the immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply, start a stopwatch and record the readings on the Voltmeter and Ammeter.#Record the time on the stopwatch with every 2°C increase in temperature a minimum of 6 times.
- Use the equation \(E = IVt\) to calculate the energy supplied to the metal block.
- Plot a graph with energy on the y-axis and temperature on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of this graph will be the mass multiplied by the specific heat capacity (mc).
Improving Accuracy
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
Improving Precision
- Use a thermometer with a higher resolution.
- Use a data logger rather than a thermometer.
Experiment Version 2a
Variables
- Independent Variable: The time over which energy is supplied to the metal block.
- Dependent Variable: The temperature of the metal block.
- Control Variables: The mass of the metal block. The power of the immersion heater.
Method
| A diagram of the apparatus used in an experiment to find the specific heat capacity of a metal block. |
- Connect an Ammeter, power supply and immersion heater in series.
- Connect a voltmeter in parallel to the immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply, start a stopwatch and record the readings on the Voltmeter and Ammeter.
- Read and record the temperature on the thermometer every 30 seconds on the stopwatch a minimum of 6 times.
- Use the equation \(E = IVt\) to calculate the energy supplied to the metal block.
- Plot a graph with energy on the y-axis and temperature on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of this graph will be the mass multiplied by the specific heat capacity (mc).
Improving Accuracy
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
Improving Precision
- Use a thermometer with a higher resolution.
- Use a data logger rather than a thermometer.