Difference between revisions of "Nuclear Fission"
(Created page with "==Key Stage 4== ===Meaning== '''Nuclear fission''' is a process in which a large unstable nucleus splits into two more Stable Isotope...") |
|||
| Line 1: | Line 1: | ||
==Key Stage 4== | ==Key Stage 4== | ||
| − | ===Meaning== | + | ===Meaning=== |
| + | [[File:NuclearFission2.png|right|300px|thumb|An [[model]] of '''nuclear fission'''.]] | ||
'''Nuclear fission''' is a process in which a large [[Unstable Isotope|unstable]] [[Atomic Nucleus|nucleus]] splits into two more [[Stable Isotope|stable]] [[Atomic Nucleus|nuclei]]. | '''Nuclear fission''' is a process in which a large [[Unstable Isotope|unstable]] [[Atomic Nucleus|nucleus]] splits into two more [[Stable Isotope|stable]] [[Atomic Nucleus|nuclei]]. | ||
| Line 9: | Line 10: | ||
: '''Nuclear fission''' can be induced in a [[material]] by bombarding [[massive]] [[Atomic Nucleus|nuclei]] with [[neutron]]s. If a [[neutron]] is captured by the [[Atomic Nucleus|nucleus]] it becomes so unstable that it splits in two. | : '''Nuclear fission''' can be induced in a [[material]] by bombarding [[massive]] [[Atomic Nucleus|nuclei]] with [[neutron]]s. If a [[neutron]] is captured by the [[Atomic Nucleus|nucleus]] it becomes so unstable that it splits in two. | ||
: The [[neutron]]s used to induce '''fission''' must have a low [[energy]] to be captured by a [[Atomic Nucleus|nucleus]] otherwise the [[neutron]]s will just pass straight through without being captured. [[Neutron]]s with the right amount of [[energy]] to be captured are called [[Thermal Neutron|thermal neutron]]s because they have a similar [[energy]] to [[molecule]]s in the [[air]] at [[Room Temperature|room temperature]]. | : The [[neutron]]s used to induce '''fission''' must have a low [[energy]] to be captured by a [[Atomic Nucleus|nucleus]] otherwise the [[neutron]]s will just pass straight through without being captured. [[Neutron]]s with the right amount of [[energy]] to be captured are called [[Thermal Neutron|thermal neutron]]s because they have a similar [[energy]] to [[molecule]]s in the [[air]] at [[Room Temperature|room temperature]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
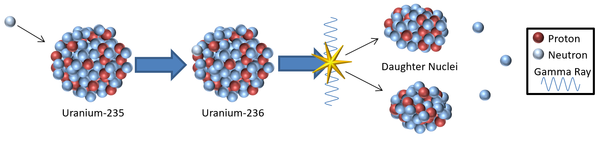
| + | |[[File:InducedFission.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:600px; text-align:left;" |A [[model]] showing a possible mechanism for induced '''nuclear fission'''. | ||
| + | |} | ||
Revision as of 15:43, 10 March 2019
Key Stage 4
Meaning
File:NuclearFission2.png
An model of nuclear fission.
Nuclear fission is a process in which a large unstable nucleus splits into two more stable nuclei.
About Nuclear Fission
- Nuclear fission occurs when a massive nucleus is so unstable that it splits in two.
- During nuclear fission neutrons are also emitted.
- Nuclear fission transfers energy from the nuclear potential energy store into the thermal energy store of the material and the surroundings.
- Nuclear fission can be induced in a material by bombarding massive nuclei with neutrons. If a neutron is captured by the nucleus it becomes so unstable that it splits in two.
- The neutrons used to induce fission must have a low energy to be captured by a nucleus otherwise the neutrons will just pass straight through without being captured. Neutrons with the right amount of energy to be captured are called thermal neutrons because they have a similar energy to molecules in the air at room temperature.
| A model showing a possible mechanism for induced nuclear fission. |