Difference between revisions of "Ionising Radiation"
(→Key Stage 4) |
(→Ionising Atoms) |
||
| Line 35: | Line 35: | ||
|} | |} | ||
| + | {| class="wikitable" | ||
| + | |- | ||
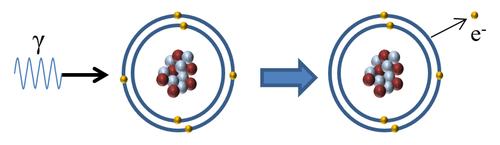
| + | |[[File:GammaIonise.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:center;" |When a [[Gamma Ray|gamma ray]] interacts with an [[atom]] the [[Gamma Ray|gamma ray]] is [[Absorb (Physics)|absorbed]] by an [[electron]] in the [[Outer Shell|outer shell]] causing the [[electron]] to escape '''ionising''' the [[atom]]. | ||
| + | |} | ||
===Ionising Radiation and Cancer=== | ===Ionising Radiation and Cancer=== | ||
: Exposure to '''ionising radiation''' is a [[Risk Factor|risk factor]] in [[cancer]]. | : Exposure to '''ionising radiation''' is a [[Risk Factor|risk factor]] in [[cancer]]. | ||
Revision as of 12:29, 7 March 2019
Contents
Key Stage 3
Meaning

The hazard symbol for ionising radiation.
Ionising Radiation is radiation which can cause atoms to lose electrons and become ions.
About Ionising Radiation
- Ionising radiation damages living organisms.
- Ionising radiation may kill cells by damaging the parts inside them, particularly the DNA.
- Ionising radiation can cause the appearance of burns to the skin. A high enough dose of Ionising Radiation can cause instant death.
Key Stage 4
Meaning
Ionising Radiation is radiation emitted from the nucleus of an atom which can cause other atoms to lose electrons and become ions.
About Ionising Radiation
- The units of exposure to ionising radiation are the Sievert which is 1 Joule of energy from ionising radiation being absorbed by 1 kilogram of flesh.
In Nuclear Physics there are three types of ionising radiation:
- Alpha Radiation - The emission of an alpha particle from the nucleus of an unstable isotope.
- Beta Radiation - The emission of a beta particle from the nucleus of an unstable isotope.
- Gamma Radiation - The emission of a gamma ray from the nucleus of an unstable isotope.
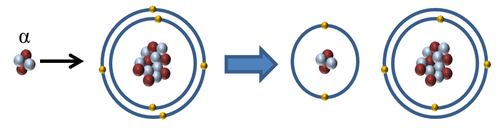
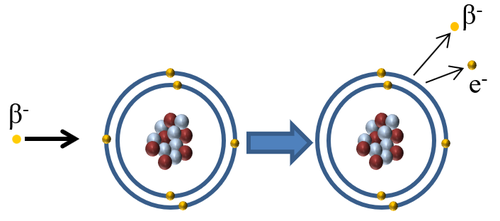
Ionising Atoms
| When an alpha particle interacts with an atom the alpha particle can remove one or two electrons to ionise the atom. |
| When a Beta minus particle interacts with an atom the beta minus particle can pass on some of its kinetic energy to an electron in the outer shell causing the electron to escape ionising the atom. |
| When a gamma ray interacts with an atom the gamma ray is absorbed by an electron in the outer shell causing the electron to escape ionising the atom. |
Ionising Radiation and Cancer
- Exposure to ionising radiation is a risk factor in cancer.