Difference between revisions of "Flame Emission Spectroscopy"
(Created page with "==Key Stage 4== ===Meaning=== right|300px|thumb|The [[Line Spectrum|line spectra from the '''flame emission spectroscopy''' of several [...") |
|||
| (4 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
==Key Stage 4== | ==Key Stage 4== | ||
===Meaning=== | ===Meaning=== | ||
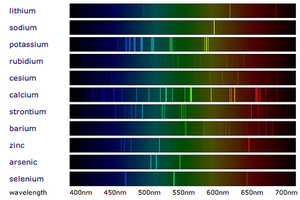
| − | [[File:FlameEmissionSpectroscopy.png|right|300px|thumb|The [[ | + | [[File:FlameEmissionSpectroscopy.png|right|300px|thumb|The [[Emission Spectra|line spectra]] from the '''flame emission spectroscopy''' of several [[metal]]s.]] |
| − | '''Flame emission spectroscopy''' is a technique for identifying [[metal]]s in a [[ | + | '''Flame emission spectroscopy''' is a technique for identifying [[metal]]s in a [[metal]] [[compound]]. |
===About Flame Emission Spectroscopy=== | ===About Flame Emission Spectroscopy=== | ||
: '''Flame emission spectroscopy''' is an advanced version of the [[Flame Test]]s and uses a [[Spectroscope]] to separate the colours into a [[spectrum]]. | : '''Flame emission spectroscopy''' is an advanced version of the [[Flame Test]]s and uses a [[Spectroscope]] to separate the colours into a [[spectrum]]. | ||
| − | : When [[White Light|white light]] passes through a [[Spectroscope]] the colours are split into a [[spectrum]] (the rainbow). When [[Metal Compound|metal compounds]] burn they only produce certain colours so when this [[light]] is passed through a [[spectroscope]] in '''flame emission spectroscopy''' it produces very specific lines called a '[[Line Spectrum]]' instead of the broad [[spectrum]] seen from [[White Light|white light]]. | + | : When [[White Light|white light]] passes through a [[Spectroscope]] the colours are split into a [[spectrum]] (the rainbow). When [[Metal Compound|metal compounds]] burn they only produce certain colours so when this [[light]] is passed through a [[spectroscope]] in '''flame emission spectroscopy''' it produces very specific lines called a '[[Emission Spectra|Line Spectrum]]' instead of the broad [[spectrum]] seen from [[White Light|white light]]. |
| − | : [[Metal]]s in a [[ | + | : [[Metal]]s in a [[metal]] [[compound]] can be identified by comparing it to the [[Emission Spectra|line spectra]] of known [[metal]]s. |
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d126 ''Flame emission spectroscopy, page 90, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Flame emission spectroscopy, pages 214-15, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Flame emission spectroscopy, pages 262, 263, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Flame emission spectroscopy, pages 263, 284-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Latest revision as of 16:11, 15 November 2019
Key Stage 4
Meaning
Flame emission spectroscopy is a technique for identifying metals in a metal compound.
About Flame Emission Spectroscopy
- Flame emission spectroscopy is an advanced version of the Flame Tests and uses a Spectroscope to separate the colours into a spectrum.
- When white light passes through a Spectroscope the colours are split into a spectrum (the rainbow). When metal compounds burn they only produce certain colours so when this light is passed through a spectroscope in flame emission spectroscopy it produces very specific lines called a 'Line Spectrum' instead of the broad spectrum seen from white light.
- Metals in a metal compound can be identified by comparing it to the line spectra of known metals.
References
AQA
- Flame emission spectroscopy, page 90, GCSE Chemistry; The Revision Guide, CGP, AQA
- Flame emission spectroscopy, pages 214-15, GCSE Chemistry, Hodder, AQA
- Flame emission spectroscopy, pages 262, 263, GCSE Chemistry, CGP, AQA
- Flame emission spectroscopy, pages 263, 284-5, GCSE Chemistry; Student Book, Collins, AQA