Difference between revisions of "Polymerisation"
(→Examples) |
|||
| (4 intermediate revisions by one other user not shown) | |||
| Line 6: | Line 6: | ||
[[Polymerisation]] may happen between: | [[Polymerisation]] may happen between: | ||
*Identical [[monomer]]s - [[Alkene]]s to Polyalkenes | *Identical [[monomer]]s - [[Alkene]]s to Polyalkenes | ||
| − | *Two different [[monomer]]s with complimantary [[Functional Group|functional groups]] at each end. - | + | *Two different [[monomer]]s with complimantary [[Functional Group|functional groups]] at each end. - Esters to Polyesters |
*Several different [[monomer]]s of a [[Homologous Series|homologous series]] - [[Peptide]]s to [[Polypeptide]]s. | *Several different [[monomer]]s of a [[Homologous Series|homologous series]] - [[Peptide]]s to [[Polypeptide]]s. | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|[[File:StructuralDiagramEthene.png|center|125px]] | |[[File:StructuralDiagramEthene.png|center|125px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:PolytheneFormula.png|center|200px]] | |[[File:PolytheneFormula.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Ethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an | + | | style="height:20px; width:200px; text-align:center;" |[[Ethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an [[Addition Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Polythene]] (sometimes spelled Polyethene) is formed. | | style="height:20px; width:200px; text-align:center;" |[[Polythene]] (sometimes spelled Polyethene) is formed. | ||
| Line 24: | Line 24: | ||
|- | |- | ||
|[[File:StructuralDiagramTetrafluoroethene.png|center|125px]] | |[[File:StructuralDiagramTetrafluoroethene.png|center|125px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:PolyTetraFluoroEtheneFormula.png|center|200px]] | |[[File:PolyTetraFluoroEtheneFormula.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Tetrafluoroethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an | + | | style="height:20px; width:200px; text-align:center;" |[[Tetrafluoroethene]] [[monomer]]s can [[Chemical Reaction|react]] together in an [[Addition Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Polytetrafluoroethene]] (sometimes referred to as PTFE or by | + | | style="height:20px; width:200px; text-align:center;" |[[Polytetrafluoroethene]] (sometimes referred to as PTFE or by the trademark Teflon<sup>TM</sup>) is formed. |
|} | |} | ||
| Line 35: | Line 35: | ||
|- | |- | ||
|[[File:StructuralDiagramPropene.png|center|200px]] | |[[File:StructuralDiagramPropene.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:PolyPropeneFormula.png|center|200px]] | |[[File:PolyPropeneFormula.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Propene]] [[monomer]]s can [[Chemical Reaction|react]] together in an | + | | style="height:20px; width:200px; text-align:center;" |[[Propene]] [[monomer]]s can [[Chemical Reaction|react]] together in an [[Addition Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Polypropene]] is formed. | | style="height:20px; width:200px; text-align:center;" |[[Polypropene]] is formed. | ||
| Line 46: | Line 46: | ||
|- | |- | ||
|[[File:StructuralDiagramEthandioateEthandiol.png|center|200px]] | |[[File:StructuralDiagramEthandioateEthandiol.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:StructuralDiagramPolyester.png|center|200px]] | |[[File:StructuralDiagramPolyester.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |Ethandioate and Ethandiol can [[Chemical Reaction|react]] together in a | + | | style="height:20px; width:200px; text-align:center;" |Ethandioate and Ethandiol can [[Chemical Reaction|react]] together in a [[Condensation Polymerisation]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |A [[Polyester]] is formed along with [[Water]]. | | style="height:20px; width:200px; text-align:center;" |A [[Polyester]] is formed along with [[Water]]. | ||
| Line 57: | Line 57: | ||
|- | |- | ||
|[[File:StructuralDiagramGlucose.png|center|200px]] | |[[File:StructuralDiagramGlucose.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:StructuralDiagramStarch.png|center|200px]] | |[[File:StructuralDiagramStarch.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Glucose]] [[molecule]]s [[Chemical Reaction|react]] together in a | + | | style="height:20px; width:200px; text-align:center;" |[[Glucose]] [[molecule]]s [[Chemical Reaction|react]] together in a [[Condensation Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |[[Starch]] is formed along with [[Water]]. | | style="height:20px; width:200px; text-align:center;" |[[Starch]] is formed along with [[Water]]. | ||
| Line 68: | Line 68: | ||
|- | |- | ||
|[[File:StructuralDiagramGlycine.png|center|200px]] | |[[File:StructuralDiagramGlycine.png|center|200px]] | ||
| − | |[[File:ArrowRight.png|center| | + | |[[File:ArrowRight.png|center|100px]] |
|[[File:StructuralDiagramPolyglycine.png|center|200px]] | |[[File:StructuralDiagramPolyglycine.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |[[Glycine]] [[molecule]]s [[Chemical Reaction|react]] together in a | + | | style="height:20px; width:200px; text-align:center;" |[[Glycine]] [[molecule]]s [[Chemical Reaction|react]] together in a [[Condensation Polymerisation]] [[Chemical Reaction|reaction]]. |
| | | | ||
| style="height:20px; width:200px; text-align:center;" |A [[Polypeptide]] ([[Protein]]) is formed along with [[Water]]. In reality [[Polypeptide]]s are made of many different [[Peptide]]s ([[Amino Acid]]s) rather than the same one repeated. | | style="height:20px; width:200px; text-align:center;" |A [[Polypeptide]] ([[Protein]]) is formed along with [[Water]]. In reality [[Polypeptide]]s are made of many different [[Peptide]]s ([[Amino Acid]]s) rather than the same one repeated. | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Polymerisation; addition, pages 184-185, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Polymerisation; condensation, pages 188-189, GCSE Chemistry, Pearson, Edexcel ''] | ||
Latest revision as of 12:53, 27 November 2019
Contents
Key Stage 4
Meaning
Polymerisation is a chemical reaction in which small molecules known as monomers react to form a polymer.
About Polymerisation
Polymerisation may happen between:
- Identical monomers - Alkenes to Polyalkenes
- Two different monomers with complimantary functional groups at each end. - Esters to Polyesters
- Several different monomers of a homologous series - Peptides to Polypeptides.
Examples
| Ethene monomers can react together in an Addition Polymerisation reaction. | Polythene (sometimes spelled Polyethene) is formed. |
| Tetrafluoroethene monomers can react together in an Addition Polymerisation reaction. | Polytetrafluoroethene (sometimes referred to as PTFE or by the trademark TeflonTM) is formed. |
| Propene monomers can react together in an Addition Polymerisation reaction. | Polypropene is formed. |
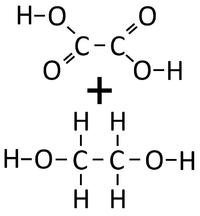
| Ethandioate and Ethandiol can react together in a Condensation Polymerisation. | A Polyester is formed along with Water. |
| Glucose molecules react together in a Condensation Polymerisation reaction. | Starch is formed along with Water. |
| Glycine molecules react together in a Condensation Polymerisation reaction. | A Polypeptide (Protein) is formed along with Water. In reality Polypeptides are made of many different Peptides (Amino Acids) rather than the same one repeated. |