Difference between revisions of "Positive Ion"
(Created page with "==Key Stage 4== ===Meaning=== '''Positive ions''' are elements which have lost one or more electrons to become positively charged. ===About Positi...") |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
===About Positive Ions=== | ===About Positive Ions=== | ||
: In [[Chemical Reaction|chemical reactions]] between [[metal]]s and [[non-metal]]s the [[metal]] [[element]]s form '''positive ions'''. | : In [[Chemical Reaction|chemical reactions]] between [[metal]]s and [[non-metal]]s the [[metal]] [[element]]s form '''positive ions'''. | ||
| − | : [[Hydrogen]] forms '''positive ions''' in some [[compound]]s and it is these [[Hydrogen Ion|H<sup>+</sup> ions]] which can make [[solution]]s [[acid]]ic. | + | : [[Hydrogen]] forms '''positive ions''' in some [[compound]]s and it is these [[Hydrogen Ion (Chemistry)|H<sup>+</sup> ions]] which can make [[solution]]s [[acid]]ic. |
: '''Positive ions''' are attracted to [[Negative Ion|negative ions]] and to the [[Cathode|negative electrode (cathode)]] during [[electrolysis]]. | : '''Positive ions''' are attracted to [[Negative Ion|negative ions]] and to the [[Cathode|negative electrode (cathode)]] during [[electrolysis]]. | ||
| + | |||
| + | ===Examples=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:LithiumIonFormation.png|center|200px]] | ||
| + | |[[File:MagnesiumIonFormation.png|center|200px]] | ||
| + | |[[File:AluminiumIonFormation.png|center|200px]] | ||
| + | |- | ||
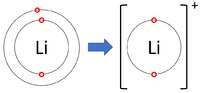
| + | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] forms +1 [[ion]]s. | ||
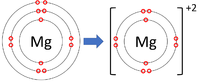
| + | | style="height:20px; width:200px; text-align:center;" |[[Magnesium]] forms +2 [[ion]]s. | ||
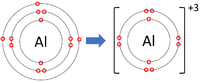
| + | | style="height:20px; width:200px; text-align:center;" |[[Aluminium]] forms +3 [[ion]]s. | ||
| + | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Positive ion, testing, pages 263, 274-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Positive ions, page 70, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Positive ions, pages 38, 186-187, 190-191, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Positive ions, pages 72, 258, 259, GCSE Chemistry, CGP, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Positive ions, page 95, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Positive ions, tests for, pages 196-197, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Positive ions (cations), pages 56-57, 88, 122, 125, 148-149, 272, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 15:45, 18 December 2019
Contents
Key Stage 4
Meaning
Positive ions are elements which have lost one or more electrons to become positively charged.
About Positive Ions
- In chemical reactions between metals and non-metals the metal elements form positive ions.
- Hydrogen forms positive ions in some compounds and it is these H+ ions which can make solutions acidic.
- Positive ions are attracted to negative ions and to the negative electrode (cathode) during electrolysis.
Examples
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |
References
AQA
- Positive ion, testing, pages 263, 274-5, GCSE Chemistry; Student Book, Collins, AQA
- Positive ions, page 70, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Positive ions, pages 38, 186-187, 190-191, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Positive ions, pages 72, 258, 259, GCSE Chemistry, CGP, AQA
Edexcel
- Positive ions, page 95, GCSE Physics, Pearson Edexcel
- Positive ions, tests for, pages 196-197, GCSE Chemistry, Pearson, Edexcel