Difference between revisions of "Plum Pudding Model"
| (2 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
: The [[Plum Pudding Model]] was proposed by [[J.J. Thompson]] who discovered the [[electron]] and realised it was part of an [[atom]]. | : The [[Plum Pudding Model]] was proposed by [[J.J. Thompson]] who discovered the [[electron]] and realised it was part of an [[atom]]. | ||
: The [[Plum Pudding Model]] was proven false by [[Rutherford's Alpha Scattering Experiment]] and was replaced by the [[Nuclear Model]]. | : The [[Plum Pudding Model]] was proven false by [[Rutherford's Alpha Scattering Experiment]] and was replaced by the [[Nuclear Model]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Plum pudding model, page 42, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe10171 ''Plum pudding model, page 104, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Plum pudding model, page 108, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Plum pudding model, page 120, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Plum pudding model, page 132, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d209 ''Plum pudding model, page 19, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Plum pudding model, page 42, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac361 ''Plum pudding model, page 43, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Plum pudding model, pages 94-95, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Plum-pudding model of the atom, pages 85, 91, GCSE Physics, Hodder, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Plum pudding model, page 149, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Plum pudding model, page 15, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Plum pudding model, page 32, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Plum pudding model, pages 78, 172, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Plum-pudding model, page 49, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Plum pudding model, page 13, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Plum pudding model, page 83, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Plum-pudding model of the atom, pages 19, Gateway GCSE Physics, Oxford, OCR ''] | ||
Latest revision as of 15:19, 18 December 2019
Contents
Key Stage 4
Meaning
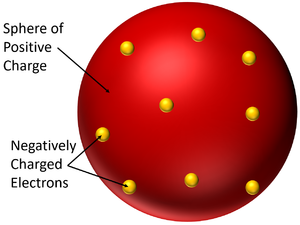
The Plum Pudding Model is a model of the atom which suggests the atom is a solid sphere of positive charge with negatively charged electrons spread within it.
About the Plum Pudding Model
- In the Plum Pudding Model the atom is neutral because the negatively charged electrons are fixed within a larger sphere of positive charge.
- The Plum Pudding Model is named after a desert made from sponge with plums stuck inside. It was imagined that the atom had a sphere of positive charge like the sponge of the cake and electrons suck inside, like the plums stuck inside the sponge.
- The Plum Pudding Model was proposed by J.J. Thompson who discovered the electron and realised it was part of an atom.
- The Plum Pudding Model was proven false by Rutherford's Alpha Scattering Experiment and was replaced by the Nuclear Model.
References
AQA
- Plum pudding model, page 42, GCSE Chemistry, CGP, AQA
- Plum pudding model, page 104, GCSE Combined Science; The Revision Guide, CGP, AQA
- Plum pudding model, page 108, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Plum pudding model, page 120, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Plum pudding model, page 132, GCSE Physics; Student Book, Collins, AQA
- Plum pudding model, page 19, GCSE Chemistry; The Revision Guide, CGP, AQA
- Plum pudding model, page 42, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Plum pudding model, page 43, GCSE Physics; The Revision Guide, CGP, AQA
- Plum pudding model, pages 94-95, GCSE Physics; Third Edition, Oxford University Press, AQA
- Plum-pudding model of the atom, pages 85, 91, GCSE Physics, Hodder, AQA
Edexcel
- Plum pudding model, page 149, GCSE Physics, CGP, Edexcel
- Plum pudding model, page 15, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Plum pudding model, page 32, GCSE Chemistry, CGP, Edexcel
- Plum pudding model, pages 78, 172, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Plum-pudding model, page 49, GCSE Physics; The Revision Guide, CGP, Edexcel